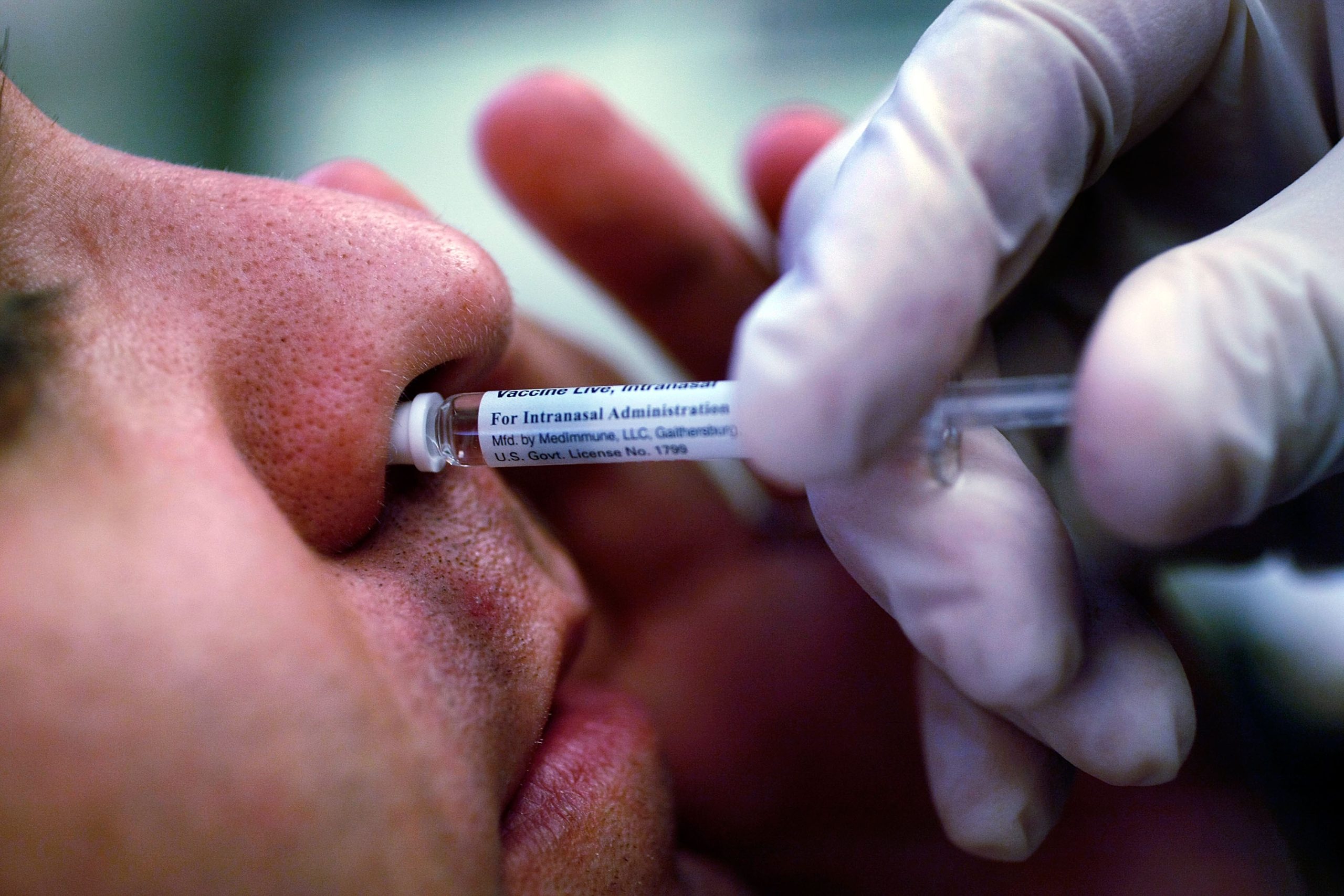
The FDA has approved a self-administered version of the FluMist nasal spray vaccine, marking the first flu vaccine that doesn’t need to be administered by a healthcare provider.
This option won’t be available until the next respiratory virus season, but it offers a new level of convenience for people who want to vaccinate themselves or their children at home. FluMist, which has been on the market since 2003, is typically used for individuals aged 2 to 49 with a prescription.
AstraZeneca, the manufacturer of FluMist, plans to make the self-administered version available through an online pharmacy. Users will go through a screening process, receive a prescription if eligible, and have the vaccine shipped directly to them. The company aims to roll out this option by the start of the next flu season, offering more flexibility for people to get vaccinated without visiting a healthcare facility.
Dr. Peter Marks from the FDA sees this development as a major step toward improving accessibility to flu vaccines. He highlights the importance of getting vaccinated every year to prevent widespread flu illnesses, which can lead to serious health complications, including hospitalizations and deaths. The self-administration option adds another tool for flu prevention, aligning with the FDA’s broader goal of advancing public health.
Despite these advancements, flu vaccination rates have been declining. In the 2023-24 flu season, there were millions of flu-related illnesses and hospitalizations, yet only around half of adults and children in the U.S. received their flu shot. FluMist offers a needle-free alternative by using a live, weakened virus, compared to injectable vaccines, which rely on killed viruses or proteins to stimulate the immune system.
Experts are divided on how much this new option will impact vaccination rates. Some believe that offering a self-administered, needle-free vaccine could encourage more people, especially those who are needle-averse, to get vaccinated.
However, the overall effect may be modest. Still, this development could increase awareness and open the door for more accessible vaccine options in the future, making it a positive step forward in flu prevention.
