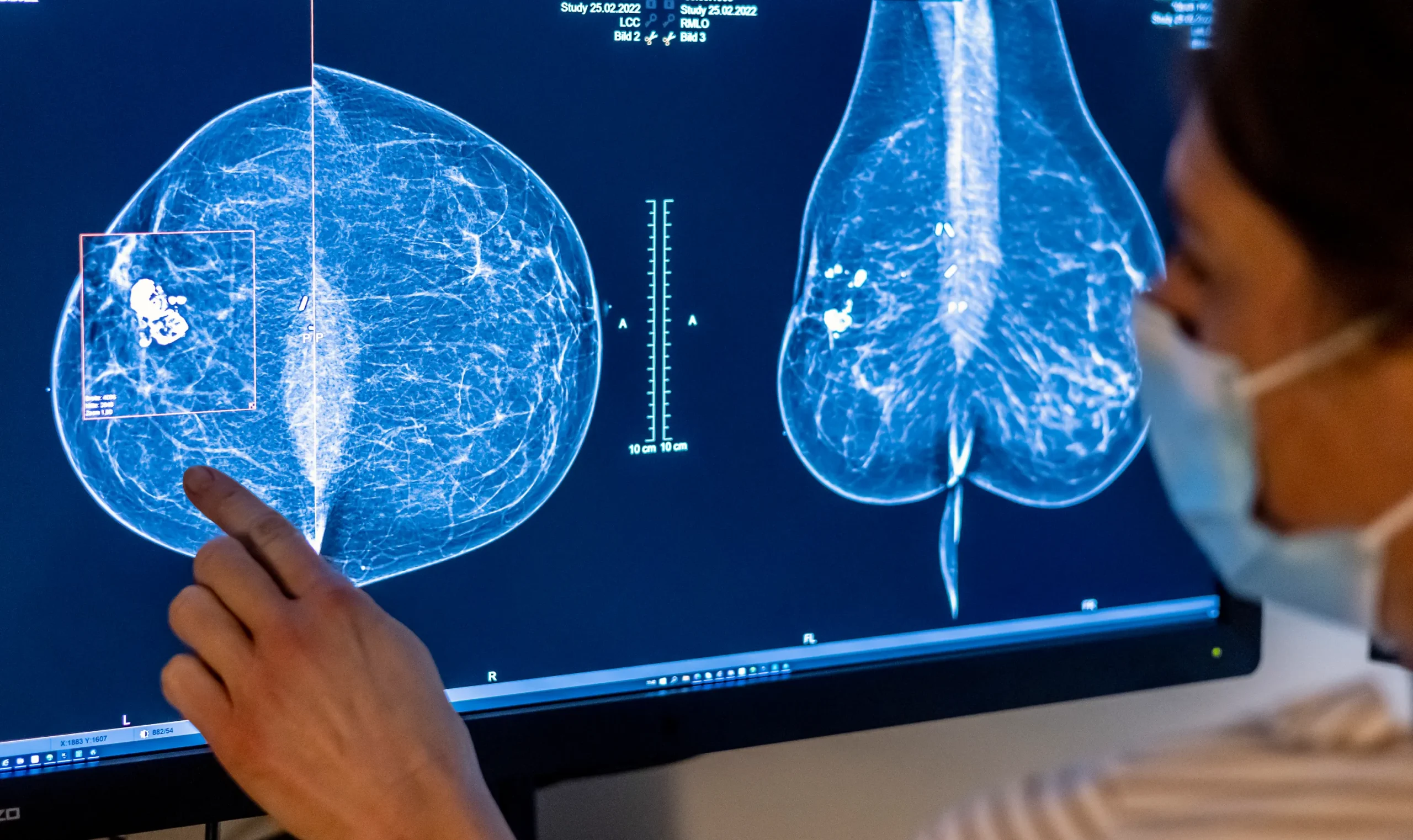
New regulations from the Food and Drug Administration (FDA) require mammogram facilities to inform patients about their breast density, effective from this week. Breast density refers to the amount of fibroglandular tissue compared to fatty tissue in the breasts.
This measure can impact the effectiveness of mammograms in detecting breast cancer, as dense breasts can obscure cancerous areas on the X-ray image. The FDA’s move is intended to provide patients with better information about their cancer risk and standardize mammogram reporting nationwide, addressing the inconsistency in state-level mandates.
Despite this advancement, there are concerns that the new rule does not fully address the issue. The regulation does not ensure that insurance companies will cover additional diagnostic tests, such as breast ultrasounds or MRIs, which are recommended for women with dense breasts.
Without insurance coverage, many patients might forego these crucial tests due to cost, which could delay the detection of breast cancer and negatively impact treatment outcomes, survival rates, and overall quality of life.
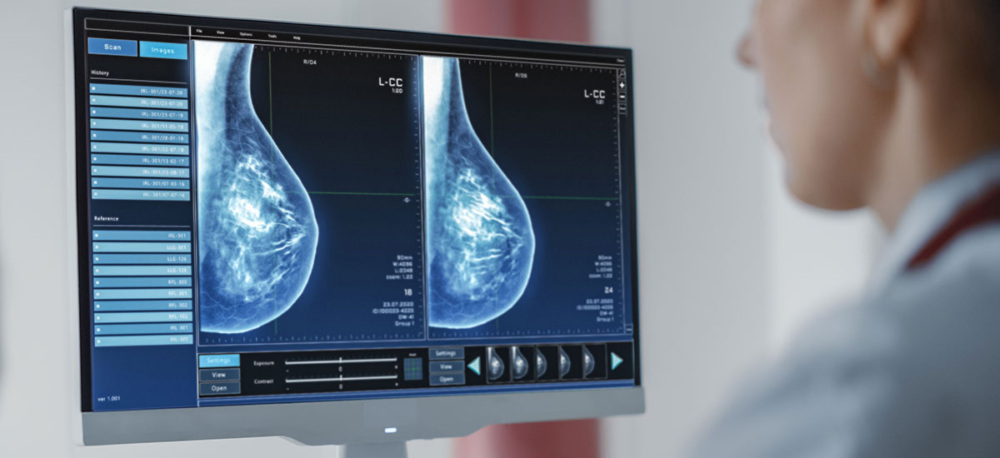
Breast density is a significant factor in cancer risk. Dense breasts, which contain more fibroglandular tissue and less fatty tissue, have a higher likelihood of developing breast cancer.
This is because fibroglandular tissue is where cancer typically forms. Additionally, mammograms are less effective in detecting cancer in dense breasts since both dense tissue and cancerous areas appear white on the X-ray, making it difficult to distinguish between them. This situation is likened to finding a snowball in a field of snow.
The new FDA rule will not alter the routine mammogram process but will provide additional information on the results. Women will receive an assessment of their breast density and an explanation of how it affects the detection of cancer. This addition aims to help patients and their healthcare providers make informed decisions about further testing and manage breast cancer risk more effectively.
Insurance coverage for supplemental tests varies significantly. While routine mammograms are covered by private insurance under the Affordable Care Act, coverage for additional diagnostic tests due to dense breast tissue is inconsistent.
Some insurance plans require prior authorization or have high out-of-pocket costs, which can delay testing and exacerbate the risk of undetected cancer spreading. This disparity highlights a gap in the healthcare system that affects many women.
Efforts are underway to address this issue through legislation. The Find It Early Act, introduced to Congress in May 2023, seeks to guarantee full insurance coverage for supplemental screening for women with dense breasts.
This bill, supported by various medical organizations and advocates, aims to improve early cancer detection and align U.S. practices with those of other high-income countries that provide more comprehensive coverage for these tests. Advocates argue that addressing this coverage gap is crucial for improving cancer outcomes and healthcare equity.