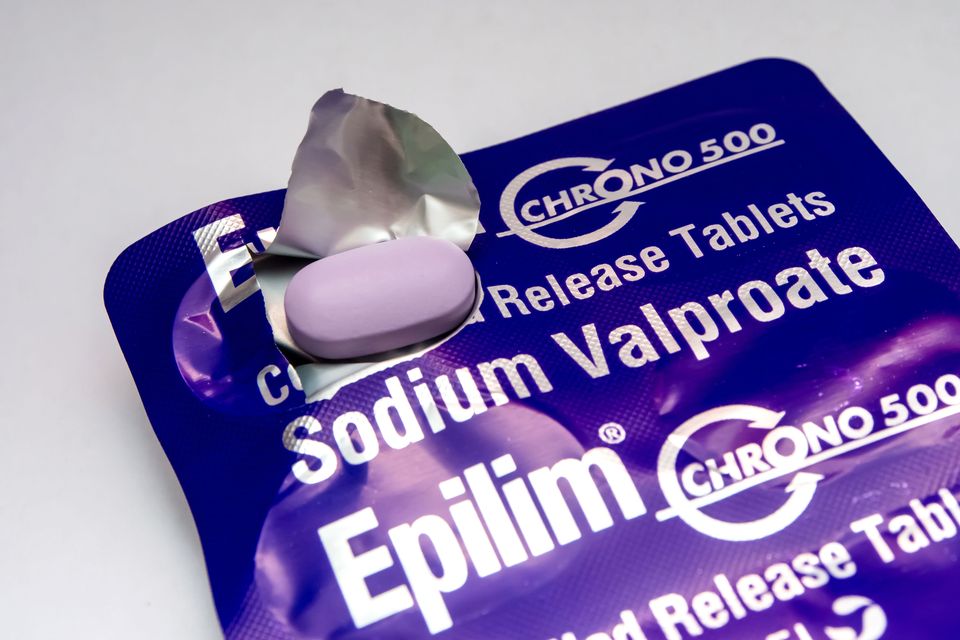
Men taking sodium valproate are now advised to use contraception while on the medication due to a “potential small increased risk” of autism and other neurodevelopmental issues in any children conceived during its use.
The warning also includes a directive to refrain from donating sperm and to continue using contraception until three months after stopping the drug.
Sodium valproate, available under brand names such as Epilim, Belvo, Convulex, and Depakote, is commonly prescribed for epilepsy and bipolar disorder.
The Medicines and Healthcare products Regulatory Agency (MHRA), which issued this warning, emphasized the importance of consulting a doctor before altering any medications.
The warning follows guidance from the European Medicines Agency after studies from Norway, Denmark, and Sweden indicated that 5% of children born to men on sodium valproate experienced developmental issues.
Although the study did not definitively prove sodium valproate as the cause, nor did it compare the outcomes with children whose fathers were not on the drug, it raised a significant “safety issue” prompting precautionary action.
Sodium valproate is also known to temporarily impair fertility, but the MHRA notes that this effect typically reverses after discontinuing the drug.
Clare Pelham, Chief Executive of the Epilepsy Society, welcomed the MHRA’s action but criticized the delay.
“It’s right for the MHRA to remain vigilant as more data about the risks of epilepsy medication emerge, and to inform the public.
But they haven’t always been proactive. People with epilepsy deserve to make informed decisions about their medication.”
Babies exposed to sodium valproate in utero face a 40% risk of developing autism or other neurodevelopmental conditions, and a 10% chance of being born with physical abnormalities.
In the UK alone, an estimated 20,000 children have suffered life-altering injuries linked to the drug.
Families in the UK and abroad have long raised concerns about the drug’s effects, often finding their fears dismissed until the European Medicines Agency issued formal warnings in 2018.
One such family is represented by the organization OACS, which supports those impacted by valproate drugs. Karen Buck, who took sodium valproate during pregnancy for epilepsy, has been vocal in her criticism of the MHRA’s slow response.
Her daughter, Bridget, now 26, suffers from Fetal Valproate Syndrome, leaving her with severe brain damage and requiring 24-hour care.
Karen expressed frustration at the MHRA’s delay in addressing the risks for men, recounting how she had requested the issue be discussed at a 2016 meeting.
“This is disgraceful. I raised this years ago. I sat with my colleagues from the charities and told the MHRA that this information needs to go out—not just for women, but for men too. I said from the start that men are being affected as well.”
In January, the MHRA advised that people under 55 should not take sodium valproate unless all other treatment options had been exhausted, and only with approval from two independent specialists. Despite this, 65,000 people under 55 in the UK still take the medication.
MHRA’s chief safety officer, Dr. Alison Cave, stressed that patients should not stop their medication without professional advice. “It is essential to attend your next appointment to discuss your treatment plan,” she said.