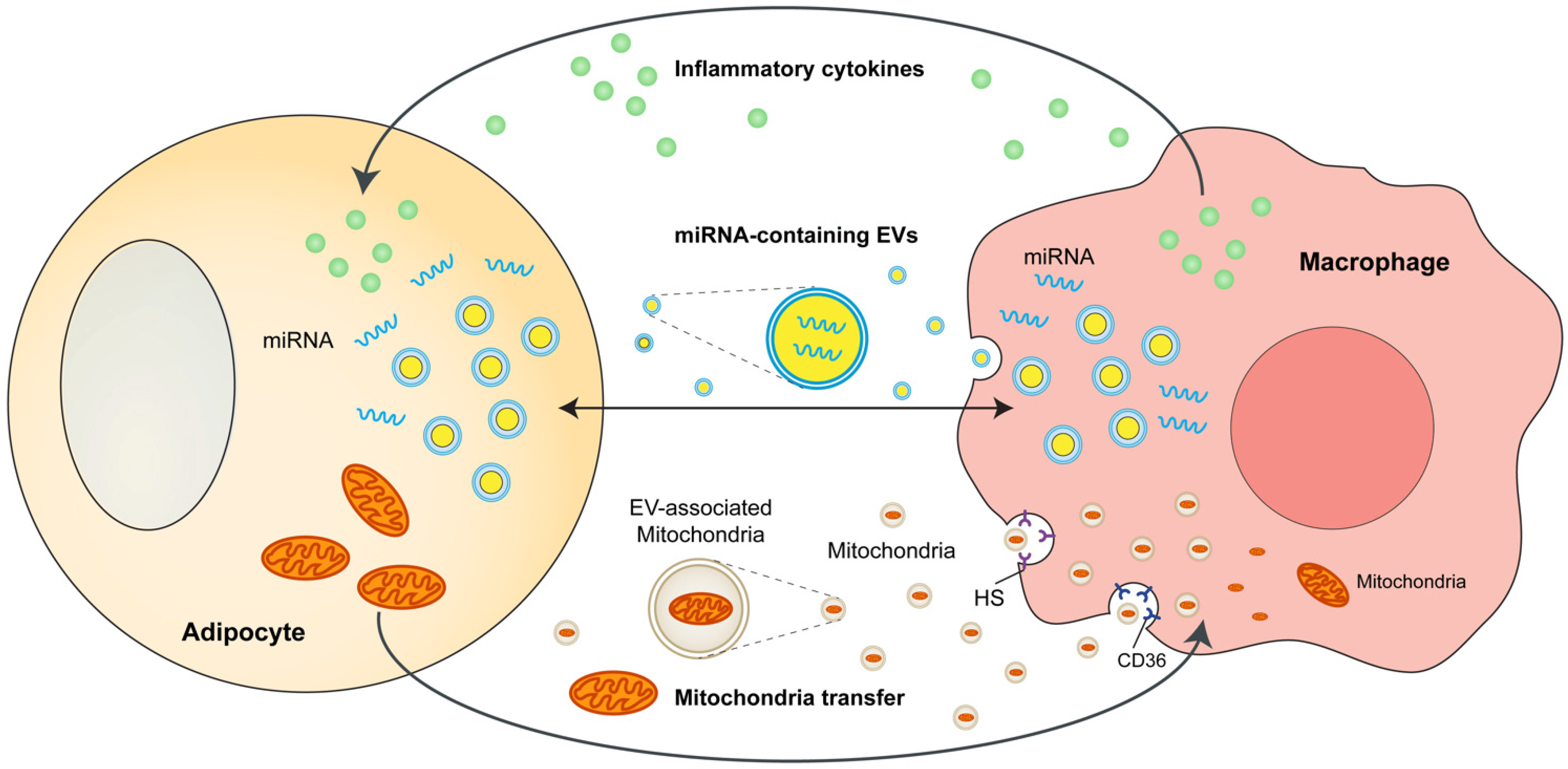
In their study published in Science China Life Sciences, Shuai Chen, Hong-Yu Wang, and colleagues explored the intricate interactions between metabolic and immune pathways, focusing on their relevance to metabolic diseases like obesity and type 2 diabetes (T2D). These conditions are increasingly prevalent globally and are rooted in the dysregulation of these pathways.
Central to their investigation was AMP-activated protein kinase (AMPK), a critical energy sensor that helps maintain metabolic homeostasis. The researchers observed that AMPK’s activity influences reactive oxygen species (ROS) production in macrophages, particularly in the context of obesity. However, the precise mechanisms linking energy status and ROS elevation in macrophages remained unclear.
Their attention then turned to Rab-GTPase activating protein (RabGAP) TBC1D1, a substrate of AMPK known to regulate ROS levels through its interaction with Rab8a. They found that a specific mutation in TBC1D1 (TBC1D1S231A) led to elevated ROS levels in macrophages, contributing to obesity-related metabolic disorders such as insulin resistance and hyperlipidemia in mice models.
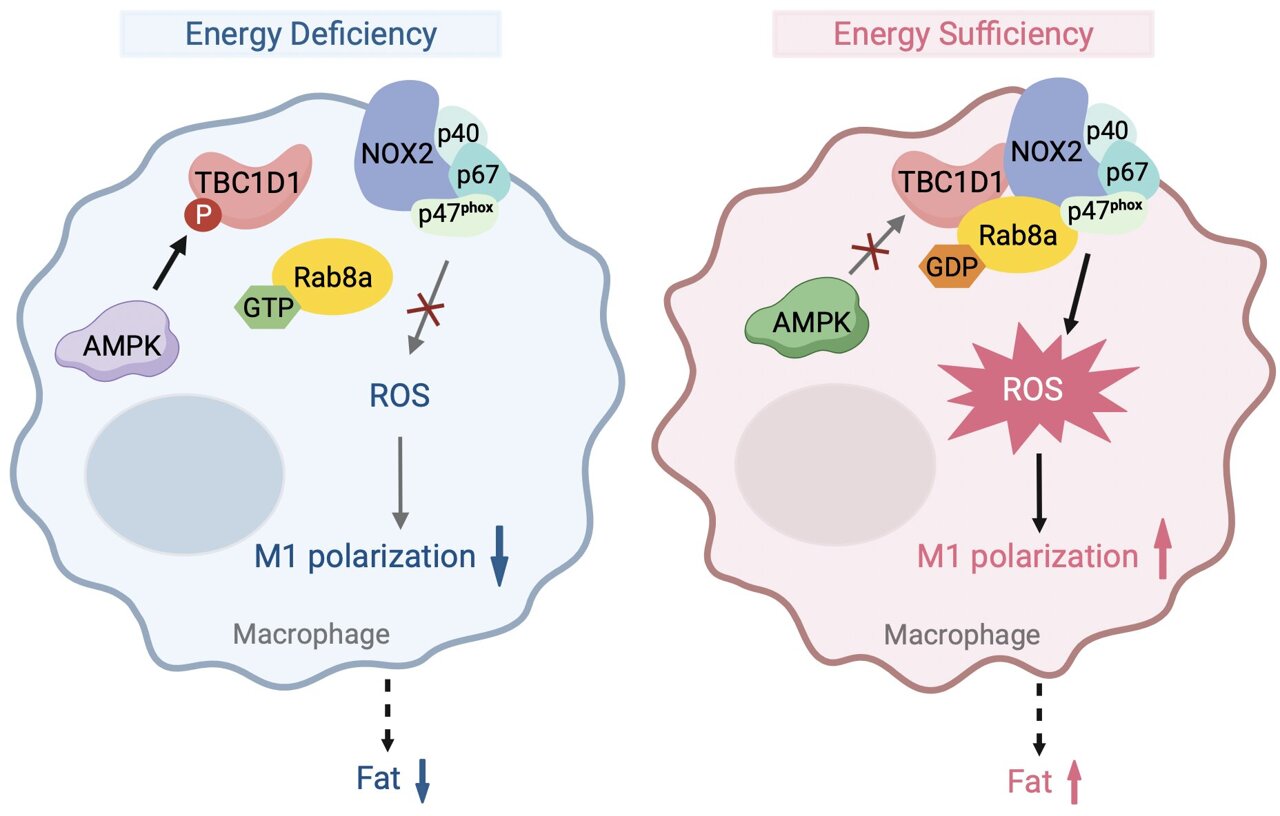
Further experiments involving bone marrow transplants and specific knockout models demonstrated that TBC1D1’s influence on ROS production directly impacted macrophage polarization. Mice lacking TBC1D1 showed reduced ROS levels and exhibited a shift towards anti-inflammatory M2-type macrophages, contrasting with the pro-inflammatory M1 polarization seen in TBC1D1S231A mutant mice.
The researchers delved deeper into the molecular mechanisms by which TBC1D1 regulates ROS production. They identified Rab8a as a critical mediator, where the GDP-bound form of Rab8a facilitated ROS generation through its interaction with NOX2 in macrophages. This pathway was shown to be pivotal in promoting inflammation associated with obesity.
To validate the role of ROS in obesity progression, the team utilized a ROS scavenger (TtSOD) targeted specifically at macrophages in adipose tissue. Treatment with TtSOD effectively mitigated obesity-related inflammation and metabolic disorders in TBC1D1S231A mice, underscoring the significance of ROS regulation in metabolic health.
This study unveils a novel regulatory pathway linking TBC1D1, Rab8a, and ROS production in macrophages under conditions of energy excess.
These findings not only deepen our understanding of metabolic-immune interactions but also suggest potential therapeutic targets for combating obesity and its associated metabolic complications. Future research inspired by these insights could pave the way for new drug discovery efforts aimed at mitigating the global burden of metabolic diseases.