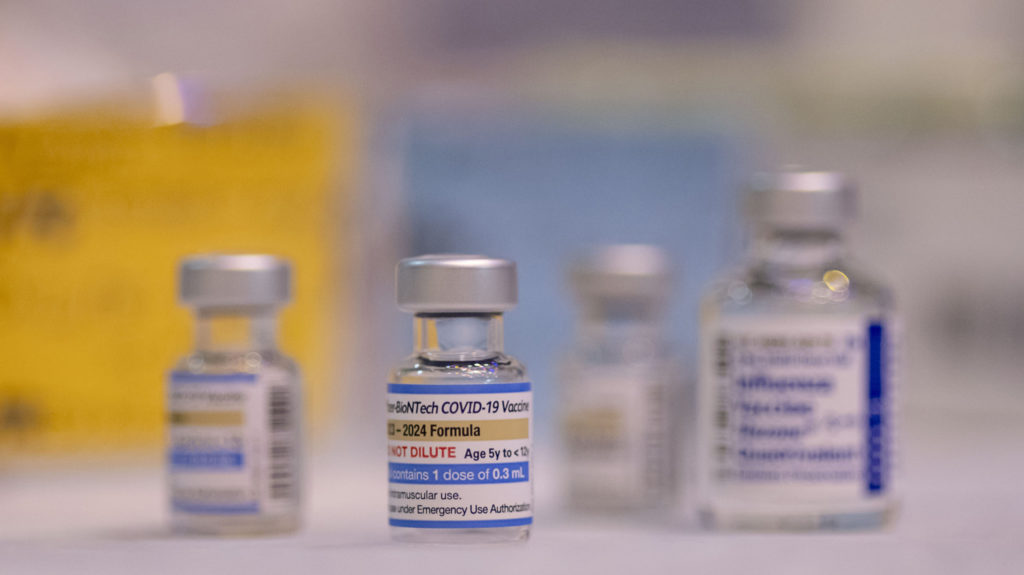
The Food and Drug Administration (FDA) on Thursday approved updated Covid-19 vaccines from Pfizer and Moderna, paving the way for the new doses to be available to most Americans within days as the country experiences a summer surge of the virus.
These updated vaccines target a strain known as KP.2, a descendant of the highly transmissible Omicron subvariant JN.1, which became widespread in the U.S. earlier this year.
Although KP.2 was the dominant strain in May, it now accounts for only about 3% of U.S. cases, according to recent data from the Centers for Disease Control and Prevention (CDC) as of Saturday.
Despite the decline of KP.2, Pfizer and Moderna have stated that their vaccines targeting this strain can elicit stronger immune responses against other circulating JN.1 subvariants, such as KP.3 and LB.1, compared to last year’s vaccines, which were designed to target the Omicron strain XBB.1.5.
“With the waning immunity in the population due to previous exposure and vaccination, we strongly encourage those eligible to consider receiving an updated COVID-19 vaccine to ensure better protection against the current variants,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
In June, the CDC recommended that everyone over 6 months old receive an updated Covid vaccine along with their flu shot this year.
The new Pfizer and Moderna vaccines are specifically approved for individuals aged 12 and older and are authorized for emergency use in children aged 6 months to 11 years.
Pfizer has announced that it will begin shipping its new vaccine immediately, with availability in pharmacies, hospitals, and clinics expected “in the coming days.” Moderna also anticipates a similar timeline for the availability of its updated vaccine.
“Staying up to date with your COVID-19 vaccine remains one of the best ways to protect yourself and prevent severe illness,” said Moderna CEO Stephane Bancel in a statement.
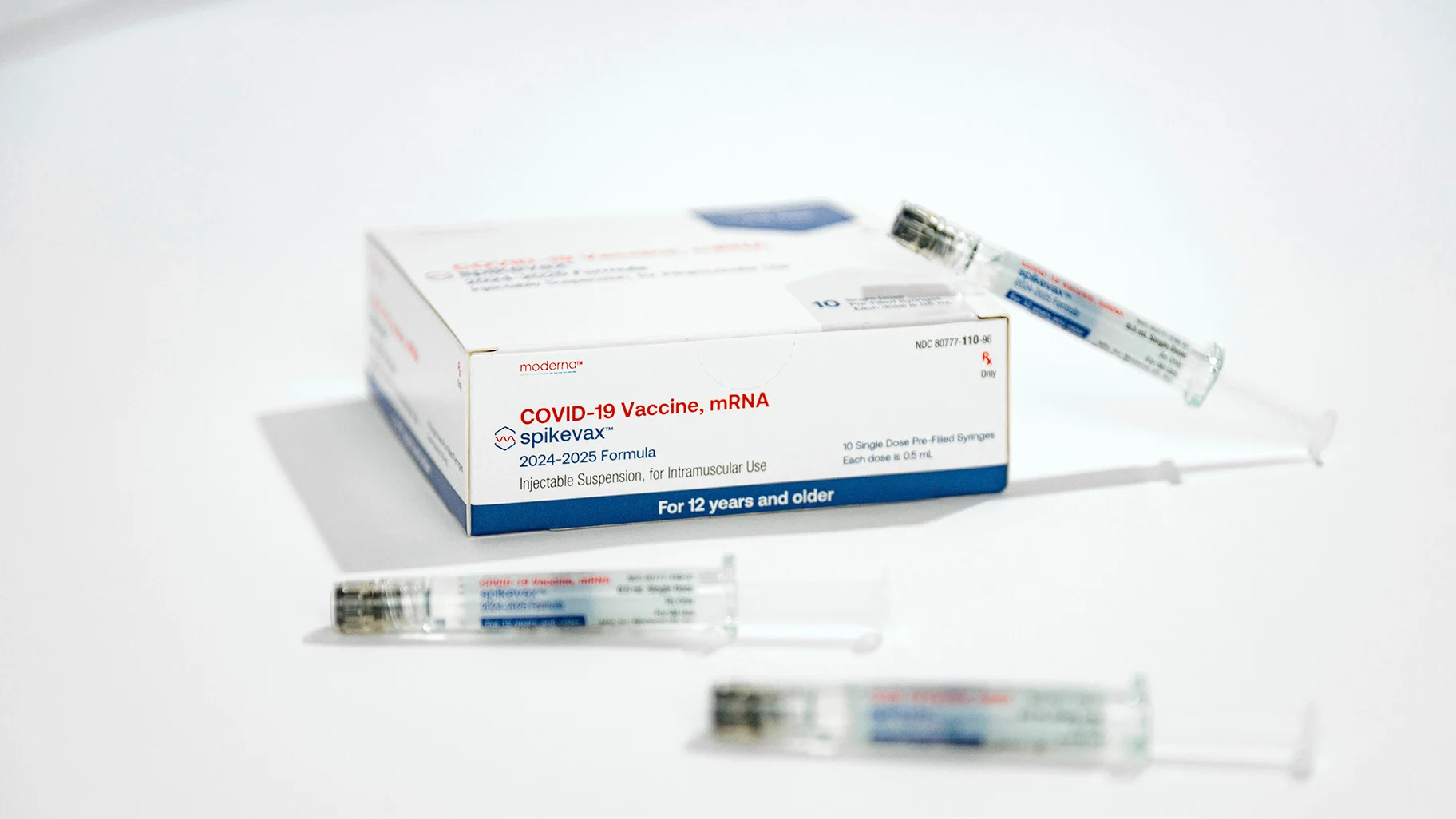
“We appreciate the U.S. FDA’s timely review and encourage individuals to talk to their healthcare providers about receiving their updated COVID-19 vaccine alongside their flu shot this fall.”
The FDA’s approval arrives a few weeks earlier than last year’s round of vaccines, which were cleared on September 11.
The earlier availability of updated vaccines could provide some reassurance to Americans as the nation faces a significant spike in Covid cases this summer.
According to CDC data, “high” or “very high” levels of Covid are being detected in wastewater in nearly every state. Wastewater monitoring offers insight into the virus’s spread as other testing methods have decreased.
Other indicators of the virus’s presence are increasing but remain significantly lower than during the peak of the pandemic. The Covid test positivity rate rose to 18.3% for the week ending August 10, up from 17.9% the previous week, according to the CDC.
Meanwhile, the CDC reports that approximately four people are being hospitalized for Covid per 100,000 individuals, up from about one hospitalization per 100,000 in May, which marked the lowest level since the pandemic began.
The summer Covid surge may subside by the time the updated vaccines reach patients and induce an immune response, typically two weeks post-vaccination.
However, federal health officials have consistently informed Americans to expect annual updates to Covid vaccines, as the virus continues to evolve and produce new strains that can evade immunity from previous vaccinations or infections, protection that diminishes over time. This process mirrors the annual rollout of flu vaccines.
It remains uncertain how many Americans will choose to receive another vaccine dose in the coming months. According to CDC data from early May, only around 22.5% of U.S. adults received the most recent round of vaccines that were released last fall.