Tens of thousands of people in England diagnosed with type 1 diabetes are poised to receive a revolutionary technology, often referred to as an artificial pancreas, to assist in managing their condition.
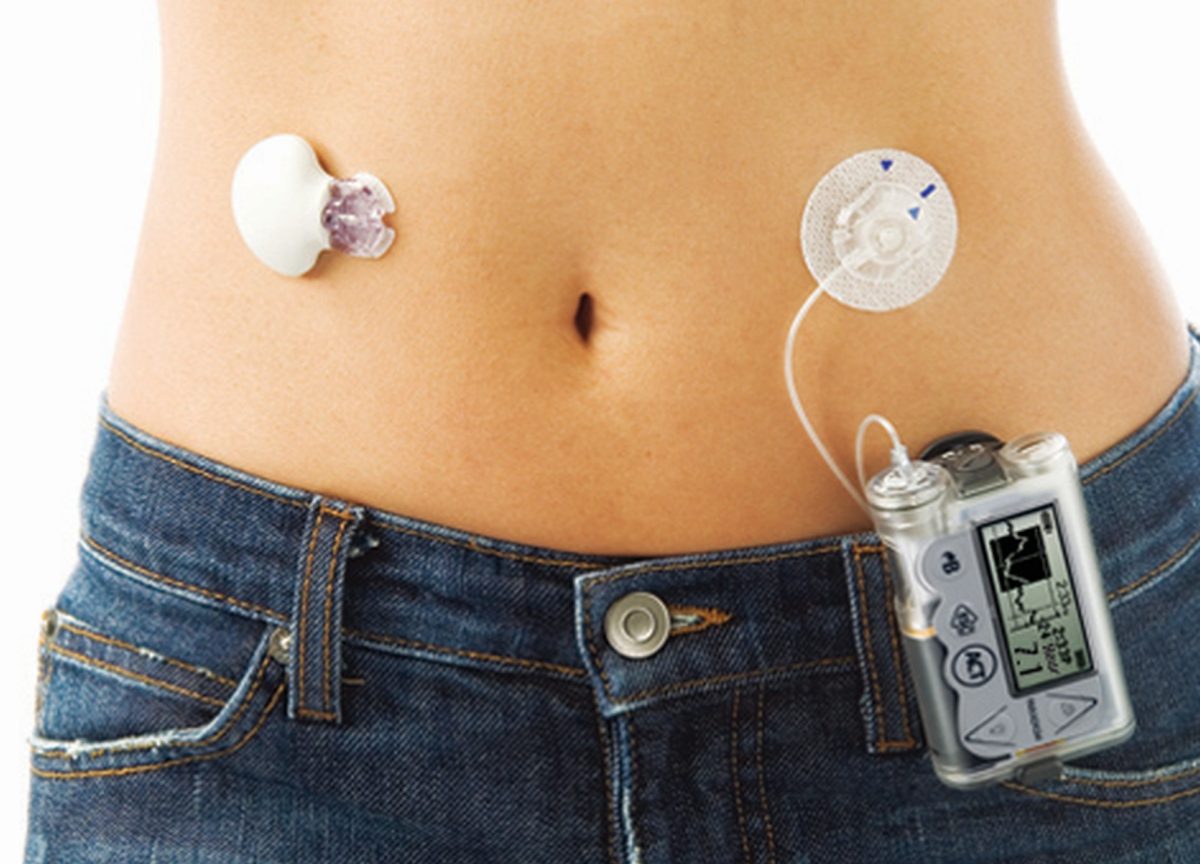
This groundbreaking system utilizes a glucose sensor positioned beneath the skin to automatically calculate the required insulin dose delivered through a pump.
Beginning later this month, the NHS will initiate contact with both adults and children who could benefit from this innovative system.
However, NHS officials caution that it may take up to five years before all eligible individuals have access.
This timeline is influenced by challenges related to sourcing an adequate supply of the devices and the necessity to train additional staff on their operation.
Trials have demonstrated that the hybrid closed-loop system significantly enhances quality of life and reduces the risk of long-term health complications.
The National Institute for Health and Care Excellence (Nice) endorsed the adoption of this technology by the NHS at the end of last year.
In the UK, nearly 300,000 individuals live with type 1 diabetes, including approximately 29,000 children. This condition arises from the pancreas failing to produce insulin, a crucial hormone responsible for converting food into energy.
Unlike traditional management methods requiring daily insulin injections or pump administration, this new technology offers automated glucose monitoring and insulin delivery, mimicking the role of a natural pancreas.

However, it still relies on inputting mealtime information for precise functionality.
The primary goal of this technological advancement is to prevent life-threatening fluctuations in blood glucose levels, which can lead to unconsciousness and, in severe cases, fatalities.
Moreover, it improves blood sugar control, thereby reducing the likelihood of complications such as heart disease, vision impairment, and kidney disease.
Scotland has already introduced this technology, and plans are underway for its implementation in Wales and Northern Ireland.
Gemma Lavery, 38, from Plymouth, who participated in an NHS pilot program, attests to its transformative impact on her daily life, eliminating stress-related glucose fluctuations and stabilizing her condition during sleep.
Prof Partha Kar, NHS national speciality advisor for diabetes, hailed the initiative as “great news for everyone with type 1 diabetes,” emphasizing its potential to enhance both medical care and quality of life.
Dr. Clare Hambling, diabetes clinical director at NHS England, echoed these sentiments, highlighting the technology’s capacity to redefine the lives of those affected by type 1 diabetes.
Colette Marshall, chief executive of Diabetes UK, expressed immense excitement about the roll-out of this transformative technology, describing it as a landmark moment in diabetes management.
Nice guidelines recommend this system for individuals with type 1 diabetes falling within specific criteria, including children and adolescents, pregnant women, and those with a HbA1c reading of 58 mmol/mol (7.5%) or higher, reflecting high long-term blood sugar levels.