Precision Neuroscience revealed on Thursday its acquisition of a Dallas-based factory aimed at advancing the development of its brain implant technology, specifically the Layer 7 Cortical Interface.
This move is expected to accelerate the company’s progress towards securing regulatory approval by 2024.
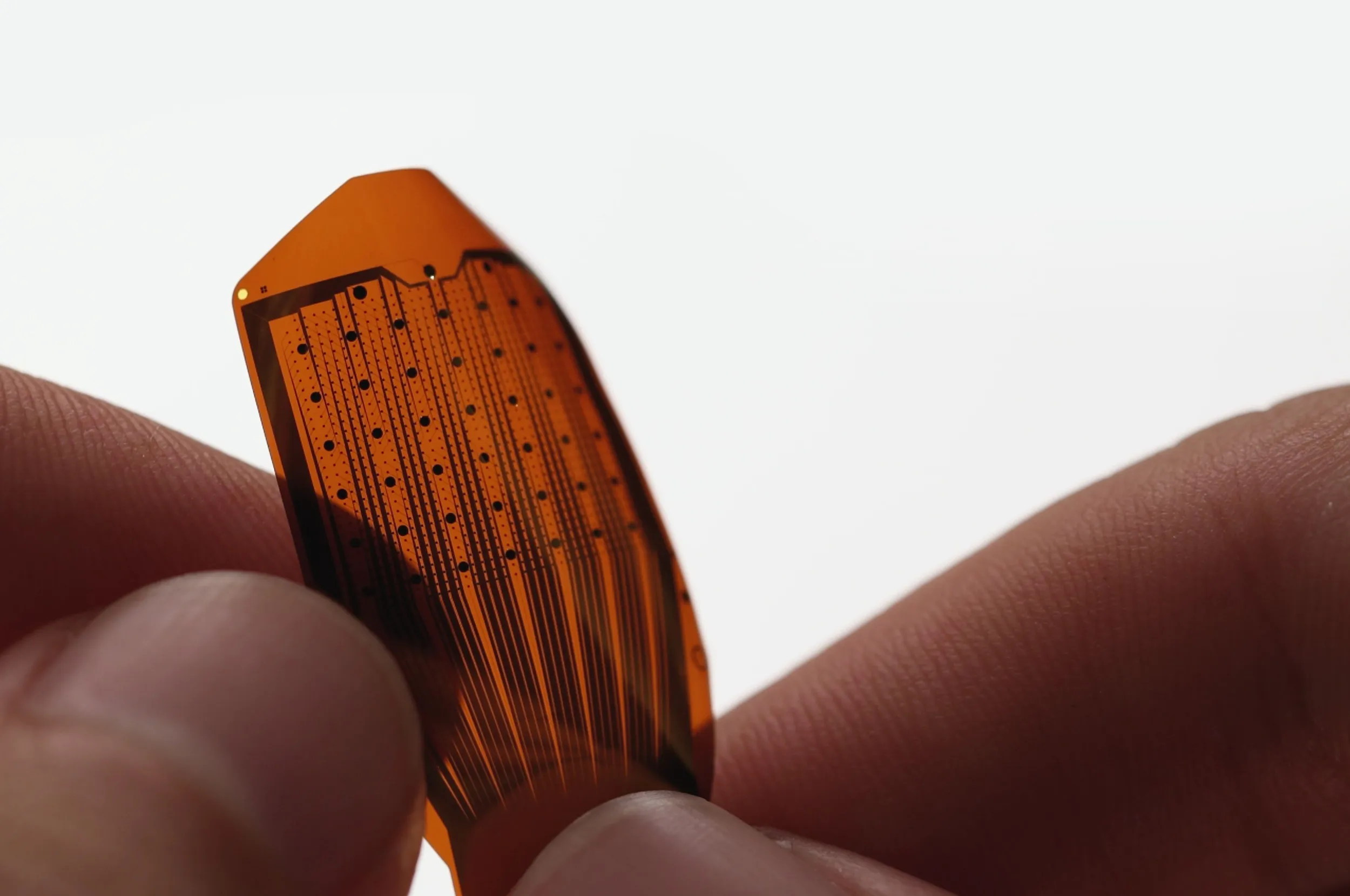
The newly acquired facility will serve as a crucial site for manufacturing Precision’s sophisticated electrode array, essential for their brain implants.
According to Michael Mager, co-founder and CEO, the facility’s integration into their operations will enable rapid iteration, enhancing device performance, longevity, and flexibility in form factors.
Precision’s electrode array, thinner than a human hair and designed akin to Scotch tape, sits on the brain’s surface without causing tissue damage, providing real-time, high-resolution neural activity monitoring.
As part of the burgeoning brain-computer interface (BCI) sector, Precision works alongside industry peers such as Synchron, Paradromics, Blackrock Neurotech, and Elon Musk’s Neuralink.
Dr. Benjamin Rapoport, Precision’s co-founder and chief science officer, was also involved with Neuralink prior to his tenure at Precision.
In contrast to Neuralink’s more invasive approach, Precision emphasizes direct involvement in manufacturing to ensure quality control and safeguard trade secrets.
Mager highlighted the challenge of maintaining supply levels and secrecy when relying on third-party manufacturers.
The acquisition of the Dallas facility, previously owned by a Japanese multinational corporation, included retaining its 11 key personnel, a strategic move that bolsters Precision’s production capabilities without significant training overhead.
Since May, the facility has already increased production significantly, a marked improvement over previous manufacturing partnerships.
The increased production capacity will support Precision’s rigorous regulatory testing, which includes forthcoming human trials at the University of Pennsylvania and the Mount Sinai Health System in New York City.
Recently, Precision received a Breakthrough Device designation from the FDA, underscoring its potential to enhance treatment for severe medical conditions.
Looking ahead, Mager expressed optimism about Precision’s trajectory, buoyed by the facility acquisition, Breakthrough Device status, and ongoing clinical trials.
He emphasized the company’s commitment to go through the complex regulatory space while advancing innovation in neurotechnology.