Research suggests that an epilepsy drug may help prevent temporary breathing stoppages in patients with sleep apnoea.
Obstructive sleep apnoea, a prevalent breathing disorder, affects roughly one in 20 people, as reported by the National Institute for Health and Care Excellence in England.
People with this condition often snore loudly, experience interrupted breathing during sleep, and may wake up multiple times during the night.
These disturbances not only cause fatigue but also increase the risk of high blood pressure, stroke, heart disease, and type 2 diabetes.
An international study has discovered that an epilepsy medication may significantly reduce the symptoms of sleep apnoea.
The results, shared at the European Respiratory Society Congress in Vienna, Austria, suggest alternative treatment options for those who struggle with mechanical breathing aids, such as continuous positive airway pressure (Cpap) machines.
Prof Jan Hedner, from Sahlgrenska University Hospital and the University of Gothenburg in Sweden, explained: “The standard treatment for obstructive sleep apnoea involves using a machine that blows air through a face mask to keep the airways open.
Unfortunately, many people find these machines difficult to use over time, so alternative treatments are necessary.”
The researchers conducted a randomised controlled trial with nearly 300 patients suffering from obstructive sleep apnoea in Belgium, the Czech Republic, France, Germany, and Spain.
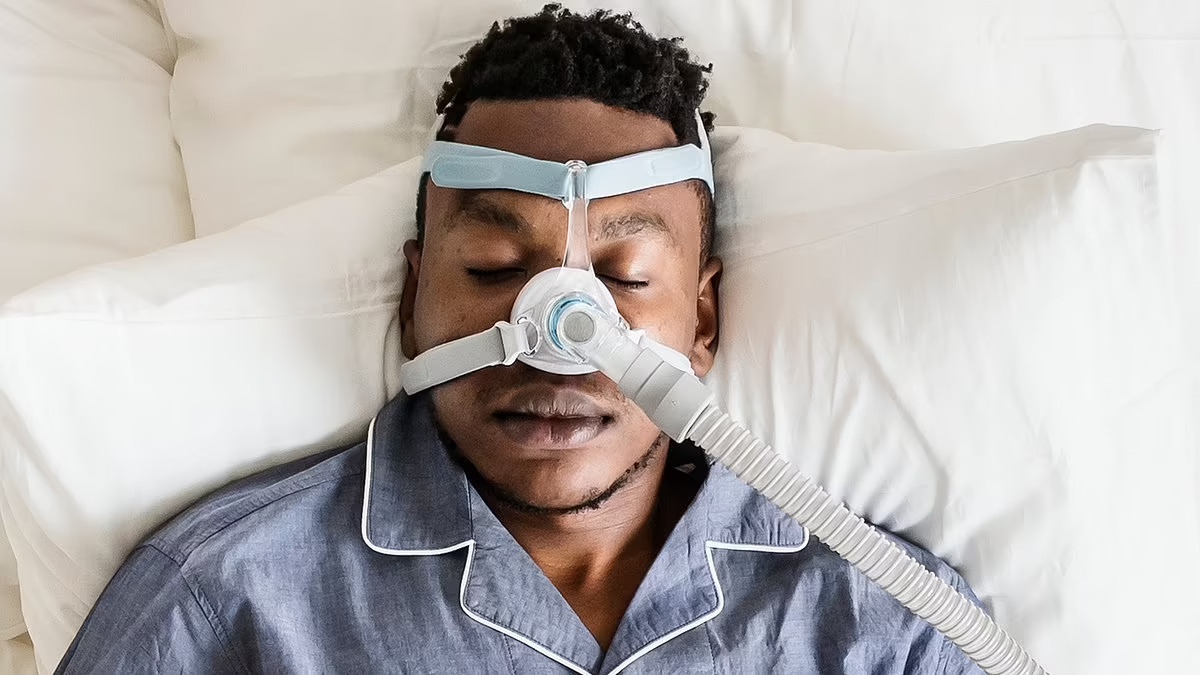
These patients were not using Cpap machines and were divided into four groups. Each group was administered one of three different doses of sulthiame or a placebo.
The study monitored participants’ breathing patterns, oxygen levels, heart rate, eye movements, and brain and muscle activity during sleep at the beginning of the trial, after four weeks, and again after 12 weeks.
After 12 weeks, those taking sulthiame experienced up to a 50% reduction in episodes of breathing cessation and showed higher oxygen levels in their blood during sleep.
The most significant improvements were observed in patients receiving the highest doses of the drug.
Hedner noted that these findings indicate sulthiame may be an effective treatment for sleep apnoea, although further research is needed to confirm its positive impact on a larger group of patients.
Erika Radford, head of health advice at Asthma + Lung UK, described the findings as an initial step toward replacing mechanical breathing aids with a medication-based treatment.
She stated, “This potential alternative to the current primary treatment could simplify condition management for patients.”
Dr. Sriram Iyer, a consultant respiratory and sleep physician and president-elect of the Royal Society of Medicine’s sleep medicine section, remarked: “This is a significant study, showing that drug therapy for sleep apnoea may soon become a reality.”
While additional research is needed to examine the long-term effects, potential side-effects, and whether certain patients would benefit more from the treatment, Iyer emphasized that it is “crucial not to overlook the fact that sleep apnoea is predominantly associated with obesity, and addressing this underlying issue should remain the priority.”
