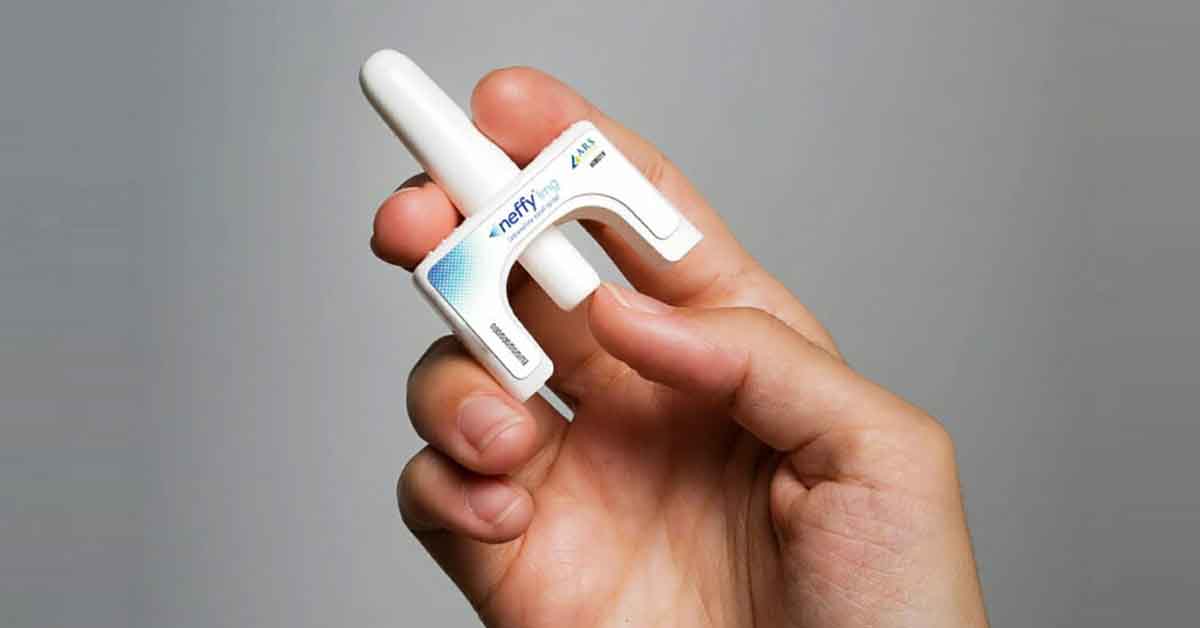
The U.S. Food and Drug Administration (FDA) has granted approval to Neffy, a groundbreaking epinephrine nasal spray designed for the emergency treatment of severe allergic reactions, including life-threatening anaphylaxis, in both adults and children. This innovative product is administered as a single dose into one nostril, with the possibility of a second dose if symptoms do not improve or worsen.
Associate Director Kelly Stone from the FDA’s Division of Pulmonology, Allergy, and Critical Care highlighted that anaphylaxis is a severe and potentially fatal condition. Many individuals, especially children, may hesitate to seek treatment due to the fear associated with injections. Neffy aims to overcome this barrier by providing a non-injection alternative, thus facilitating quicker and more accessible treatment for those in need.
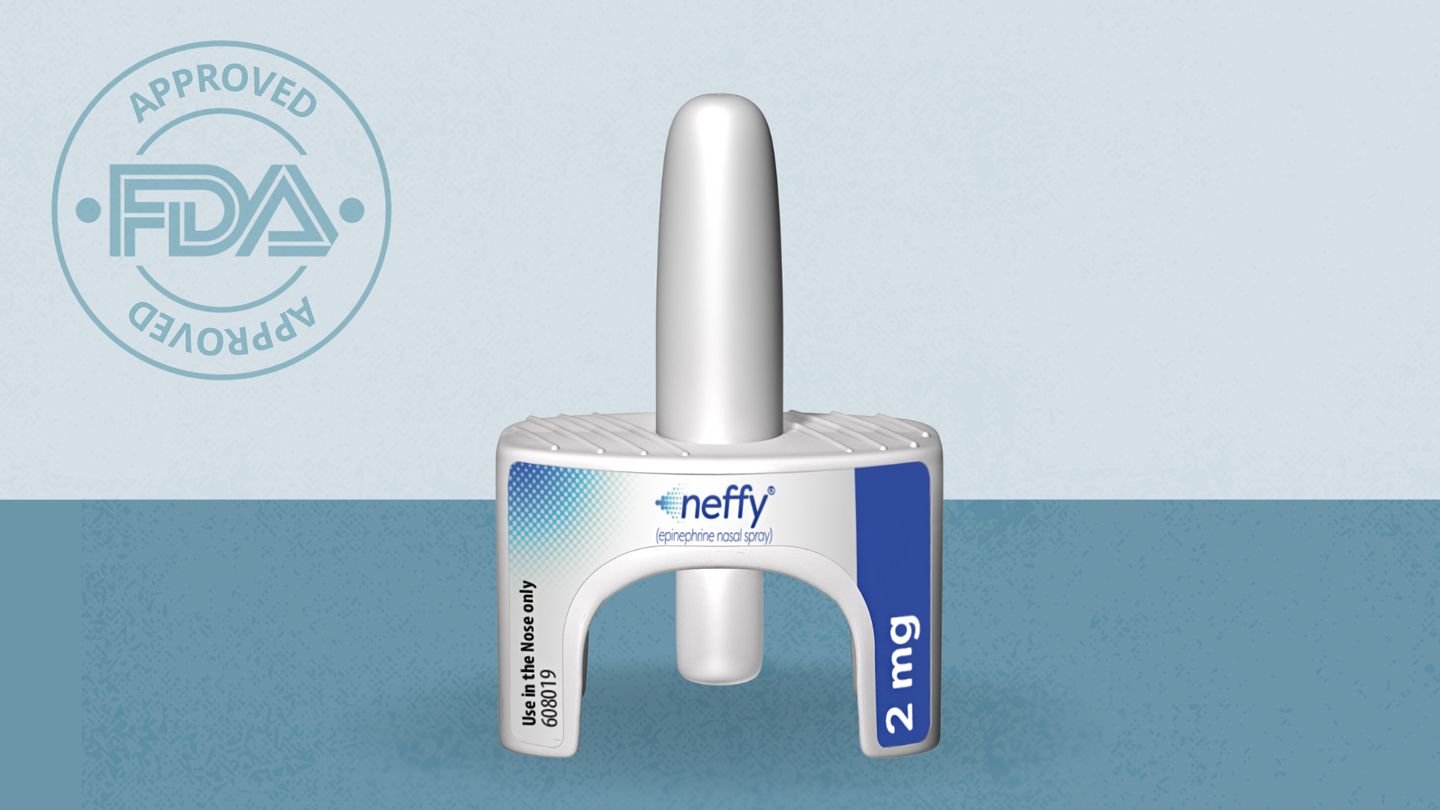
Anaphylaxis can be triggered by various allergens, including certain foods, medications, and insect stings. Traditionally, the only effective treatment for this condition is epinephrine, which is usually administered through an injection. Neffy represents a significant advancement in this area, offering a more user-friendly method of delivering epinephrine during emergencies.
The FDA’s approval of Neffy followed its Fast Track designation and endorsement for use by ARS Pharmaceuticals. This designation underscores the importance of the nasal spray in addressing a critical unmet need in the management of severe allergic reactions and demonstrates the FDA’s commitment to improving treatment options for patients experiencing anaphylaxis.