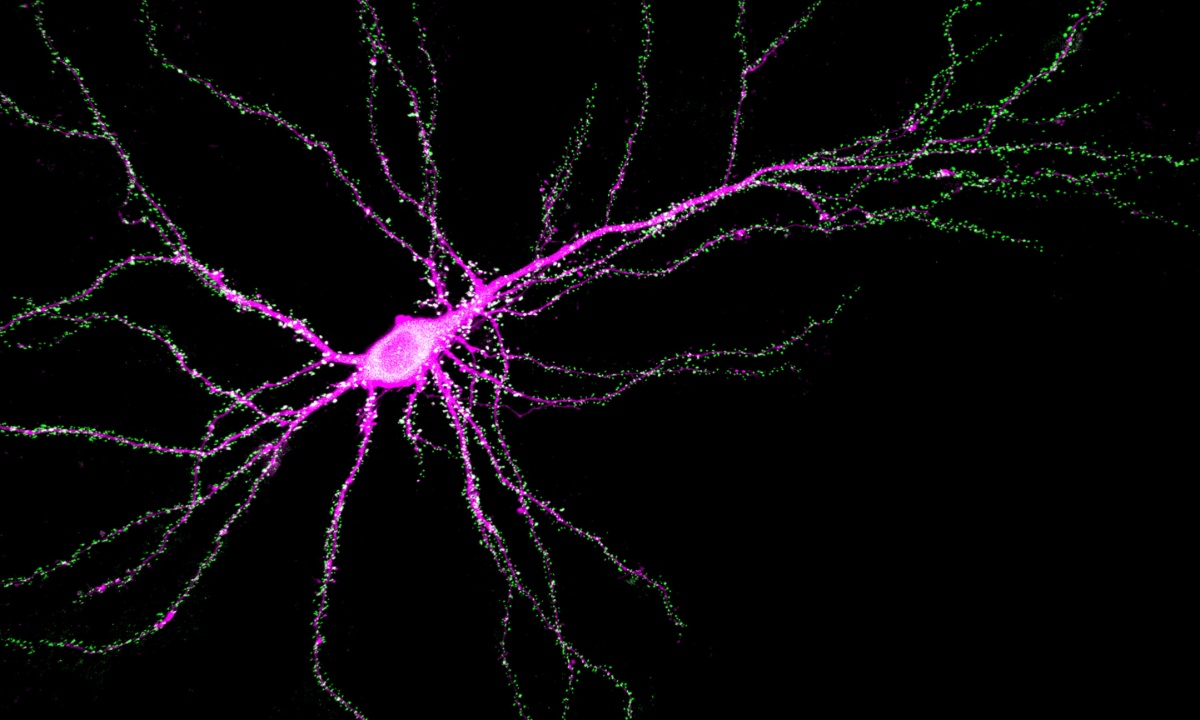
Researchers at the VIB-KU Leuven Center for Brain & Disease Research and NERF, led by Prof. Pierre Vanderhaeghen and Prof. Vincent Bonin, have uncovered that mutations in the SYNGAP1 gene disrupt the prolonged development of human neurons, a process essential for normal cognitive function.
This discovery has significant implications for understanding and developing treatments for intellectual disabilities and autism. The study is published in the journal Neuron and highlights the critical role of extended neuronal development, or neoteny, in human cognitive abilities.
Human brain development is unique among mammals, with neurons, especially in the cerebral cortex, taking years to mature. This extended developmental period is crucial for advanced cognitive functions. Disruptions in this process are suspected to underlie certain intellectual disabilities and autism.
Previous research had identified SYNGAP1 mutations as a major cause of these conditions, but their specific effects on human cortical neurons were not well understood due to the lack of reliable experimental methods to study these neurons in a living brain.

To overcome this challenge, the researchers used a xenotransplantation model, grafting human neurons with SYNGAP1 mutations into mouse brains to observe their development and function. They found that SYNGAP1 is essential for the prolonged developmental timeline of human cortical neurons.
The mutation led to an accelerated development of these neurons, establishing a link between the hastened maturation and neurodevelopmental disorders.
The study revealed that while SYNGAP1 mutant neurons appeared normal in many respects, they connected with other neurons much faster than usual, integrating into cortical circuits and responding to stimuli months ahead of schedule.
This accelerated integration and functionality suggest that early maturation of neurons could disrupt brain circuit functions and plasticity during infancy, potentially contributing to intellectual disabilities and autism.
Prof. Vanderhaeghen emphasized that the role of neoteny in normal brain development underscores the impact of its disruption on neurodevelopmental diseases. The findings could influence the diagnosis and treatment of patients with SYNGAP1 mutations and other intellectual disabilities or autism.
Prof. Bonin highlighted the significance of their transplantation model, which allows for in vivo studies of human neuronal diseases at functional and circuit levels, offering a promising avenue for understanding neurological disorders and testing new treatments.