An MIT study published in “Nature” sheds new light on the cellular and circuit vulnerabilities in Alzheimer’s disease and highlights factors that might help some individuals maintain cognitive function despite disease pathology.
By comparing gene expression across various brain regions, the researchers aimed to identify potential intervention targets that could support cognition and memory. They used a comprehensive approach involving both human brain tissue and lab experiments to validate their findings.
The study involved a large-scale analysis of gene expression, examining over 1.3 million cells from more than 70 different cell types across six brain regions. The samples came from 48 donors, with 26 diagnosed with Alzheimer’s and 22 without.
This extensive data collection provided a detailed view of how Alzheimer’s affects brain cell activity, differentiating between cell types, brain regions, and individual cognitive assessments.
The research emphasized the vulnerability of specific brain regions and cell types to Alzheimer’s. Li-Huei Tsai, co-senior author, highlighted the need to understand how particular regions or cell types are affected.
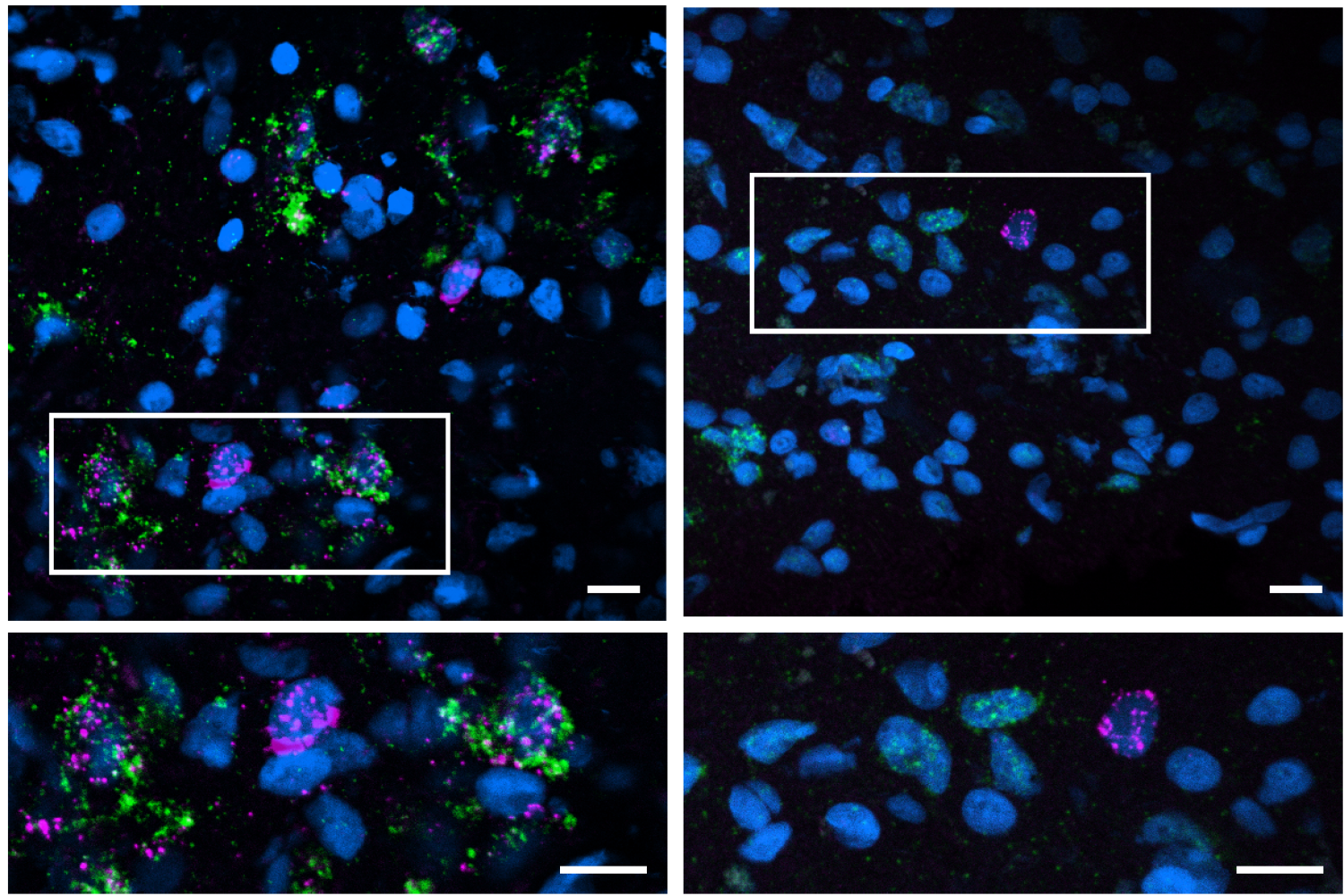
Manolis Kellis, another co-senior author, likened their advanced single-cell RNA profiling technique to a sophisticated “microscope” that reveals subtle biological changes linked to pathology, potentially offering new avenues for treatment.
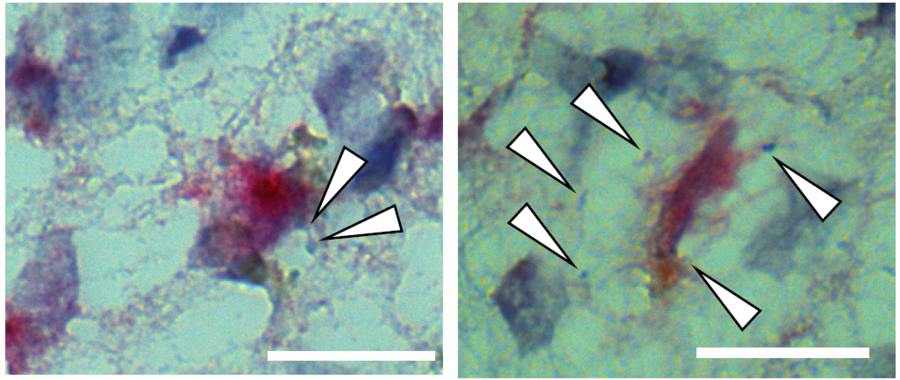
One key finding was the reduced abundance of specific excitatory neurons in the hippocampus and entorhinal cortex of Alzheimer’s patients, which correlated with poorer cognitive performance.
Many of these vulnerable neurons were found to be part of a shared neuronal circuit and were influenced by a protein called Reelin. The study suggested that Reelin might have a protective role in cognitive function, as its loss is associated with cognitive decline.
The researchers also discovered that cognitive resilience in individuals with Alzheimer’s pathology was associated with specific gene expressions in astrocytes, particularly related to choline metabolism and antioxidant activity.
These findings align with previous research on dietary supplements like choline and spermidine, which may support cognitive function by modulating these pathways.
To handle the vast amount of data, the team developed a new method focusing on gene modules, which allowed them to identify coordinated patterns of gene expression more effectively.
They have made this dataset and analytical tools publicly available, encouraging further exploration. The researchers plan to continue studying how genetic variants and regulatory factors can be manipulated to address Alzheimer’s disease at various stages and in different brain regions.