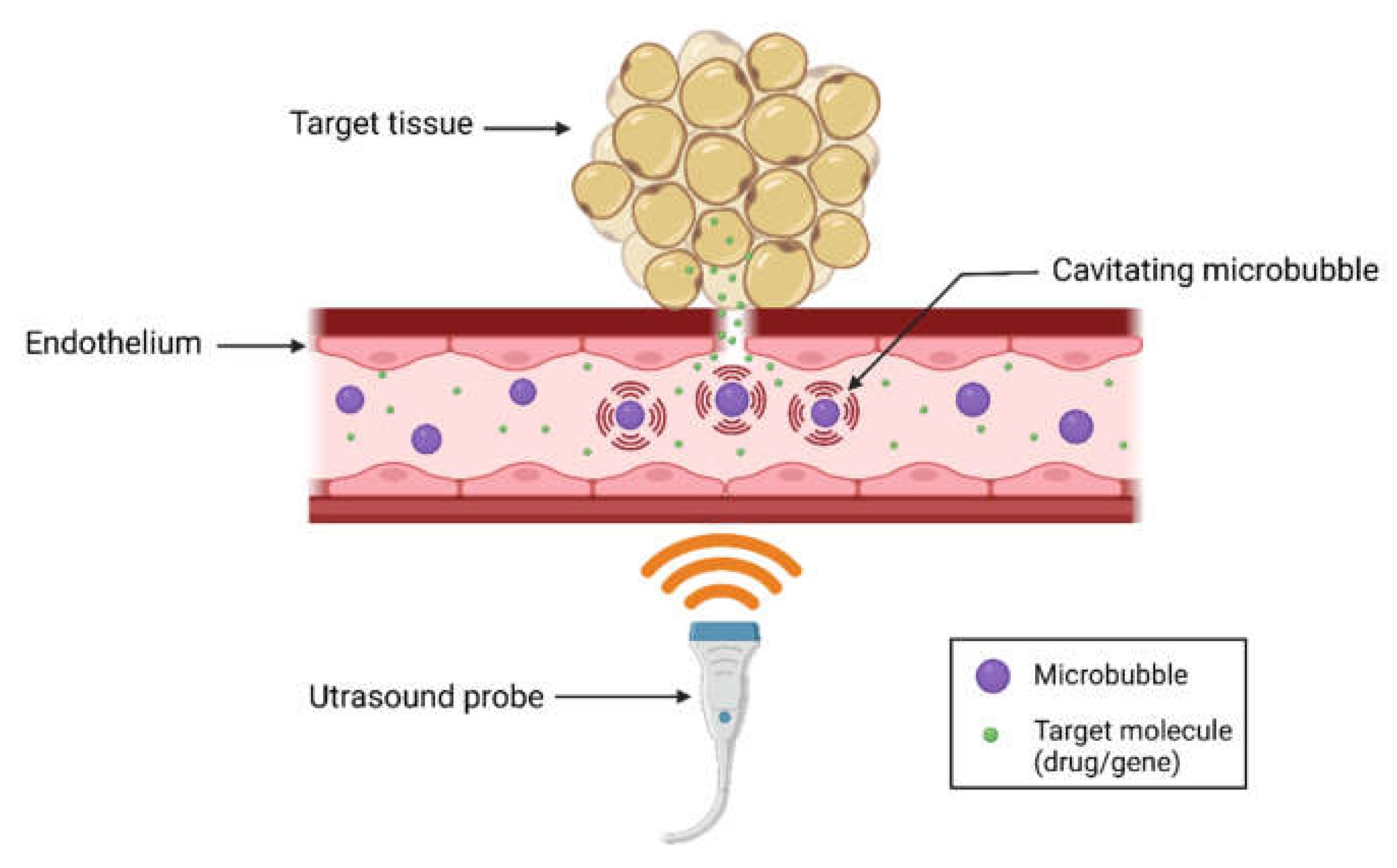
In an effort to advance targeted drug delivery, scientists from the US have developed a technique using ultrasound-triggered nanocarriers to release medications precisely where they are needed.
This approach aims to reduce the drug dose and minimize side effects. The new protocol has made this method both safe and efficient for the first time.
The researchers hope this breakthrough will lead to first-in-human trials, although the study was initially tested on a single long-tailed macaque.
Matthew G. Wilson, a graduate research assistant at the University of Utah and the study’s first author, stated:
“Here we show a method to deliver drugs to specific areas of the body where they are needed. We do so using ultrasound waves, which trigger drug release from circulating nanocarriers when focused on the target.”
He continued, “We developed a method to produce stable nanocarriers repeatably, and identified ultrasound parameters that can activate them.”
The approach involves creating stable nanocarriers with polymer shells and hydrophobic cores containing drugs.
Ultrasound waves are then used to release the drugs from these nanocarriers at precise target locations within the body.
The researchers determined the optimal ultrasound parameters and assessed the safety and efficiency of this delivery system.
The nanocarriers are tiny droplets (470-550 nanometers) with polymer shells featuring hydrophilic (water-attracting) and hydrophobic (water-repelling) ends.
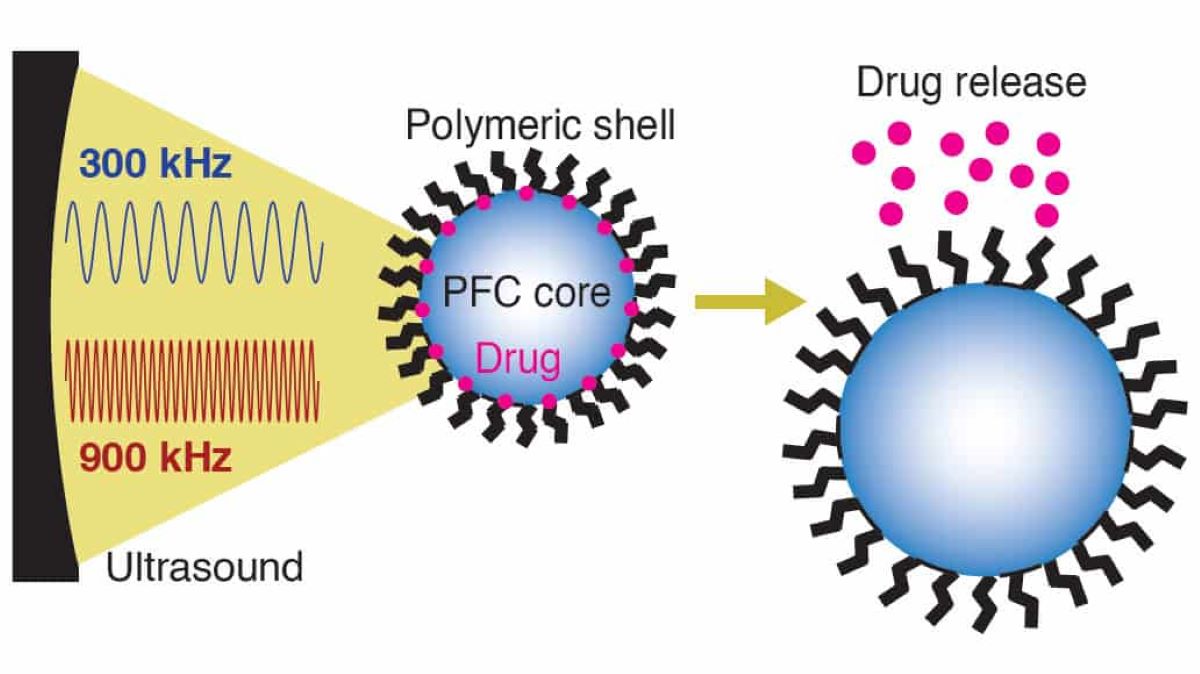
The core consists of hydrophobic perfluorocarbons mixed with a hydrophobic drug. The shell prevents the core from merging with others and shields it from the immune system.
The polymer shell ensures drug stability and targeted delivery, while ultrasound waves at 300 or 900 kilohertz make the shell permeable, allowing the drug to be released precisely at the target site.
The ultrasound causes the perfluorocarbons inside the nanocarriers to expand, making the shell more permeable and enabling the drug to diffuse to the intended area.
The researchers tested the delivery efficiency of the drug propofol using three types of perfluorocarbons (PFP, DFP, PFOB) with short pulses of ultrasound.
“Previous studies have focused on perfluorocarbons with low boiling points, usually lower than human body temperature.
We found that droplets with a PFOB core, which has a boiling point of 142°C, are much more stable over time,” Wilson explained.
“Despite its high boiling point, PFOB can achieve similar levels of drug release with low-frequency ultrasound at 300 kilohertz. The ultrasound frequency was a critical factor in our study.”
Safety testing involved administering six doses of PFOB-based nanodroplets over six weeks to a macaque. Researchers monitored blood markers for liver, kidney, and immune function.
The experiment, approved by the University of Utah’s ethics committee, revealed no side effects.
Dr. Jan Kubanek, an assistant professor at the University of Utah and the study’s senior author, noted that the method could be applied to various conditions depending on the drug used.
“For psychiatric applications, localized delivery of propofol could serve as a diagnostic tool to identify brain regions involved in disorders for individual patients. For more lasting treatment, ketamine delivery could be a potent method to rewire neural circuits.”Dr