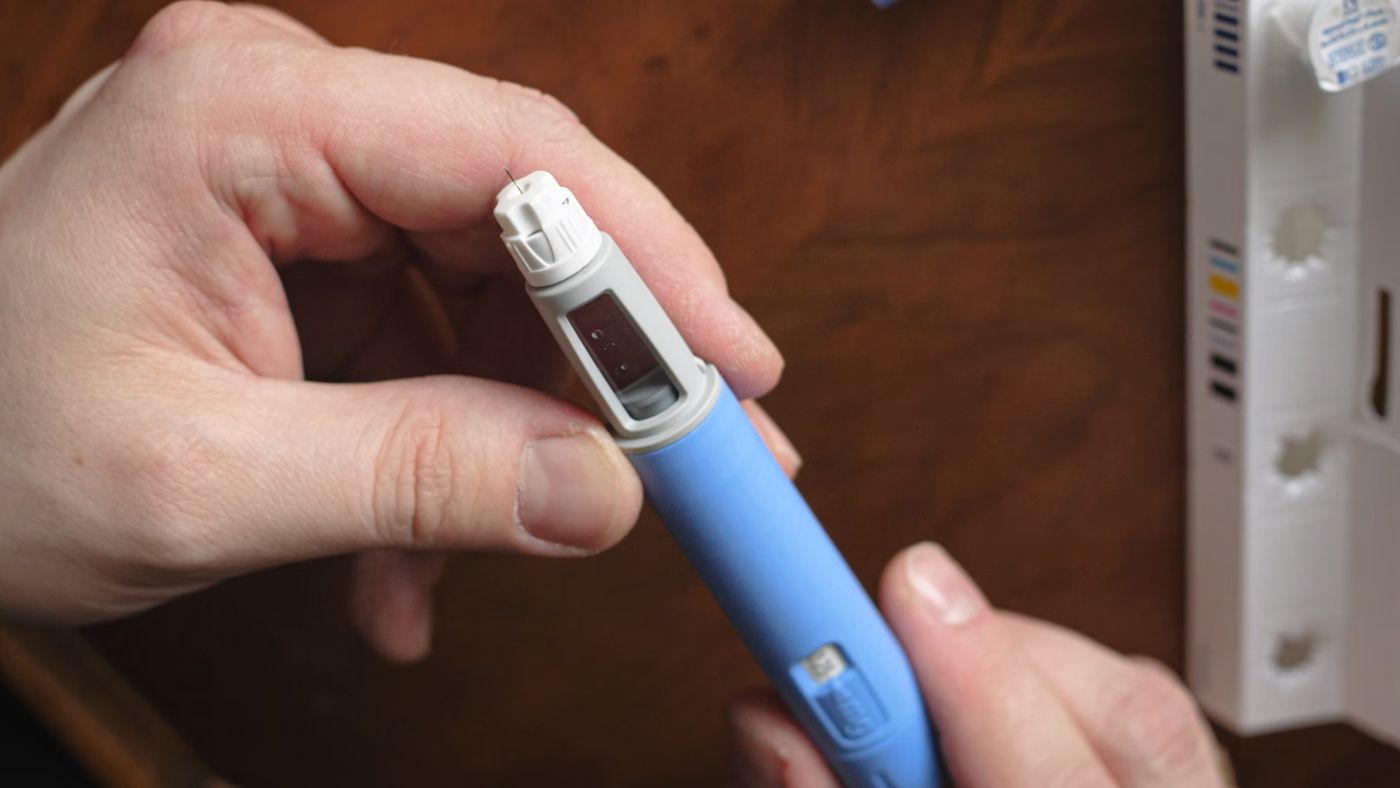
As weight loss medications gain in popularity, the Illinois Poison Center is receiving more calls related to unintentional misuse or incorrect dosing of these drugs.
“We’re definitely seeing the trend,” said Matt Novak, a trained pharmacist and certified specialist in poison information. “We’ve had some situations where people give themselves 10 times the amount that they’re supposed to take.”
In 2022, the Illinois Poison Center received 61 phone calls. In 2023, that number nearly doubled to 121. With 35 phone calls in the first quarter of 2024, calls are on track to surpass the previous year.
“You know, I think it’s just more people are taking these medications,” said Dr. Michael Wahl, medical director for the Illinois Poison Center. Wahl noted that some individuals are confused by the injector pens used to administer the medication.
“Let’s say they’re supposed to take .25 milligrams, but they set it wrong on the pen and they take 1 milligram or 4 milligrams.
They can take huge doses, make huge dosing errors, which then can lead to nausea, vomiting, diarrhea, dizziness, and that’s when they call us,” Wahl explained.
Attorney Tony Simon represents at least 25 people in Illinois with another concern.
“Some of my clients that were taking the drug had severe complications that they were never told about,” Simon said.
Simon has filed several lawsuits in Illinois against Novo Nordisk, the maker of Ozempic, claiming the drug maker did not warn about potential complications, including stomach paralysis.
“If there’s an adequate warning on the label by the manufacturer of risks that they knew about during their clinical trials, by putting it on the label and putting it in the information, that physician and their patient can adequately judge whether or not they want to do that,” Simon stated.
Novo Nordisk responded to the Illinois lawsuits with this statement:
“Novo Nordisk believes that the allegations in these lawsuits are without merit, and we intend to vigorously defend against these claims.
Patient safety is our top priority at Novo Nordisk, and we work closely with the U.S. Food and Drug Administration to continuously monitor the safety profile of our medicines.
GLP-1 medicines have been used to treat type 2 diabetes (T2D) for more than 18 years, and for the treatment of obesity for 8 years. This includes Novo Nordisk GLP-1 products such as semaglutide and liraglutide that have been on the market for more than 13 years.
Semaglutide has been extensively examined in robust clinical development programs, large real world evidence studies and has cumulatively over 9.5 million patient years of clinical experience.
The known risks and benefits of semaglutide and liraglutide medicines are described in their FDA-approved product labeling.
Novo Nordisk stands behind the safety and efficacy of all of our GLP-1 medicines when they are used as indicated and when they are taken under the care of a licensed healthcare professional.”
Rita Glaze-Rowe, president of Transformative Healthcare Markets for Real Chemistry, a healthcare innovations company with a Chicago-based office, commented on the situation.
“People cannot, don’t want to wait, until they can find a physician,” Glaze-Rowe said.
The high demand is causing shortages of some medications, with the FDA reporting limited availability for Mounjaro, Zepbound, and Wegovy.
“So what we’re seeing is people going outside of the healthcare system,” Glaze-Rowe noted.
Real Chemistry is tracking the unprecedented rise in what they call “GLP-1 influencers,” who post on social media about their experiences and drive people to med spas, telehealth providers, and compounding pharmacies.
“We were seeing that it was, ‘I can get you your semaglutide tomorrow.’ Now what we’re seeing is a little bit of a difference which says ‘it’s the same active ingredient as,” Glaze-Rowe said.
While compounded formulas of FDA-approved weight loss medications may be easier to access and cheaper, Dr. Naomi Parrella offered a warning.
“The compounded pharmacies, they do not send out the same medications that you get from a regular pharmaceutical company. So the problem is it’s not regulated.
So we don’t really know the safety profile,” said Parrella, chief of lifestyle medicine at RUSH University Medical Center.
“The FDA has actually been very clear about this. These medications should not be purchased in a compounded formula,” Parrella added.
Matt Novak, a trained pharmacist, has fielded calls at the Illinois Poison Center from people taking compounded versions.
“They are sent a vial and a syringe and patients aren’t properly trained in administering it because this is all online,” Novak explained. “They get confused and they draw up the entire vial.”
“The key is to make sure starting any medication that you get all the information up front and know how to take it safely,” Parrella emphasized.