A critical function of the immune system is to detect and eliminate cells that have developed cancerous mutations.
Despite this surveillance, early-stage cancer cells can evade detection, allowing them to progress into more advanced tumors.
A recent study conducted by MIT and Dana-Farber Cancer Institute has uncovered a mechanism that enables these precancerous cells to evade immune detection.
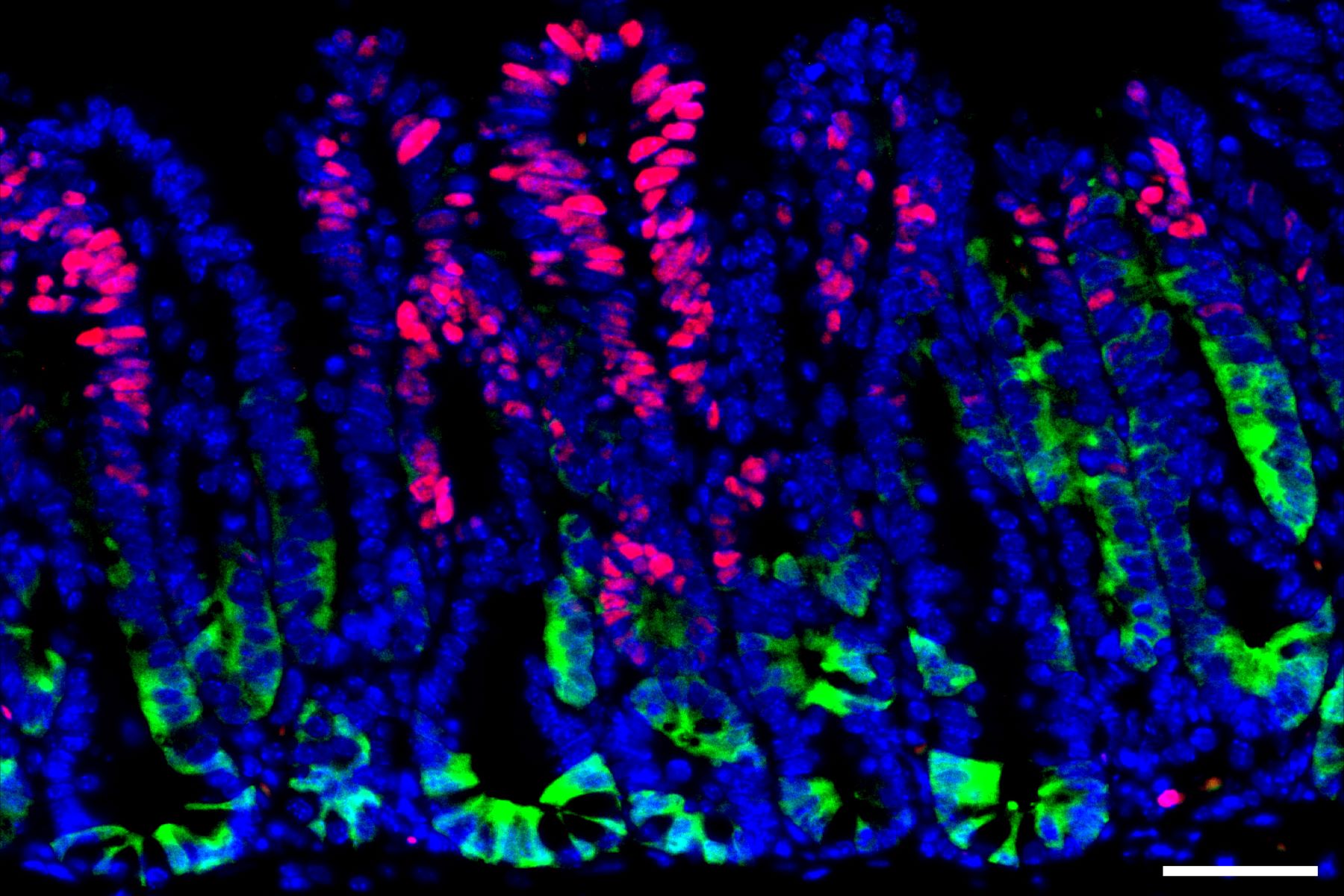
The researchers identified that during the initial stages of colon cancer development, activation of a gene called SOX17 renders these cells virtually invisible to the immune system.
Blocking the function of SOX17 or its associated pathway could potentially offer a novel approach to treating early-stage cancers before they progress into larger, more challenging tumors, the researchers propose.
“Activation of the SOX17 program in the early stages of colorectal cancer formation plays a critical role in shielding precancerous cells from immune surveillance.
Inhibiting the SOX17 program could enhance our ability to prevent colon cancer, especially in individuals predisposed to developing colon polyps,” explained Omer Yilmaz, an associate professor at MIT’s Koch Institute for Integrative Cancer Research and co-senior author of the study.
The research, published in Nature, was led by Norihiro Goto, a research scientist at MIT. Judith Agudo, a principal investigator at Dana-Farber Cancer Institute and assistant professor at Harvard Medical School, also co-led the study.
The study employed a technique previously developed by the researchers to grow miniature colon tumors in a laboratory setting and transplant them into mice.
These tumors were engineered to express mutated versions of genes (Kras, p53, and APC) commonly associated with human colon cancers.
Following implantation into mice, the researchers observed a significant increase in the expression of SOX17 within the tumors.
SOX17 is a transcription factor typically active during embryonic development, where it regulates intestinal development and blood vessel formation.
Experiments revealed that SOX17 activation in cancer cells creates an immunosuppressive microenvironment.
Specifically, it suppresses the production of receptors that normally detect interferon gamma, a key molecule in the immune system’s arsenal against cancer cells.
“In colorectal cancer cells and precancerous adenoma cells, SOX17’s role is primarily to inhibit the interferon gamma signaling pathway.
By doing so, these cells evade detection by T cells, enabling them to survive and proliferate despite the presence of an active immune system,” Yilmaz elaborated.
Furthermore, the absence of interferon gamma signaling reduces the production of MHC proteins, which are responsible for presenting cancerous antigens to the immune system.
It also impairs the production of chemokines that recruit T cells necessary for eliminating cancerous cells.
To validate their findings, the researchers generated colon tumor organoids with deactivated SOX17 and transplanted them into mice.
They observed that the immune system was much more effective in attacking these tumors, suggesting that targeting SOX17 could potentially enhance treatment outcomes for early-stage colon cancer.
“By deactivating SOX17 in complex tumors, we effectively inhibited their persistence,” Goto stated.
Analyzing gene expression data from colon cancer patients, the researchers found that SOX17 expression was notably elevated in early-stage cancers but diminished as tumors progressed to more invasive and metastatic stages.
“As colorectal cancers advance and develop additional immunosuppressive mechanisms, SOX17’s significance diminishes,” Yilmaz noted.
“Understanding these dynamics could inform future strategies for treating advanced cancers.”
The researchers acknowledge the challenges of targeting transcription factors like SOX17 with conventional drugs due to their complex structure.
Moving forward, they plan to explore interactions between SOX17 and other proteins, which may offer more feasible targets for therapeutic intervention.
Additionally, they aim to investigate the triggers responsible for activating SOX17 in precancerous cells, further elucidating the mechanisms underlying early-stage colorectal cancer development.