In 2020, biologist Michael Levin and his team made headlines by creating what they termed “biological robots” from clusters of cells that could move independently across surfaces.
These entities, named xenobots after the African clawed frog Xenopus laevis from which they were derived, sparked discussions about whether they represented a new form of organism.
This debate gained momentum when, a year later, the researchers demonstrated that xenobots could self-assemble from frog skin cells and exhibit diverse behaviors while swimming through liquids.
Critics argued that such capabilities were not unexpected in amphibian cells, known for their regenerative prowess
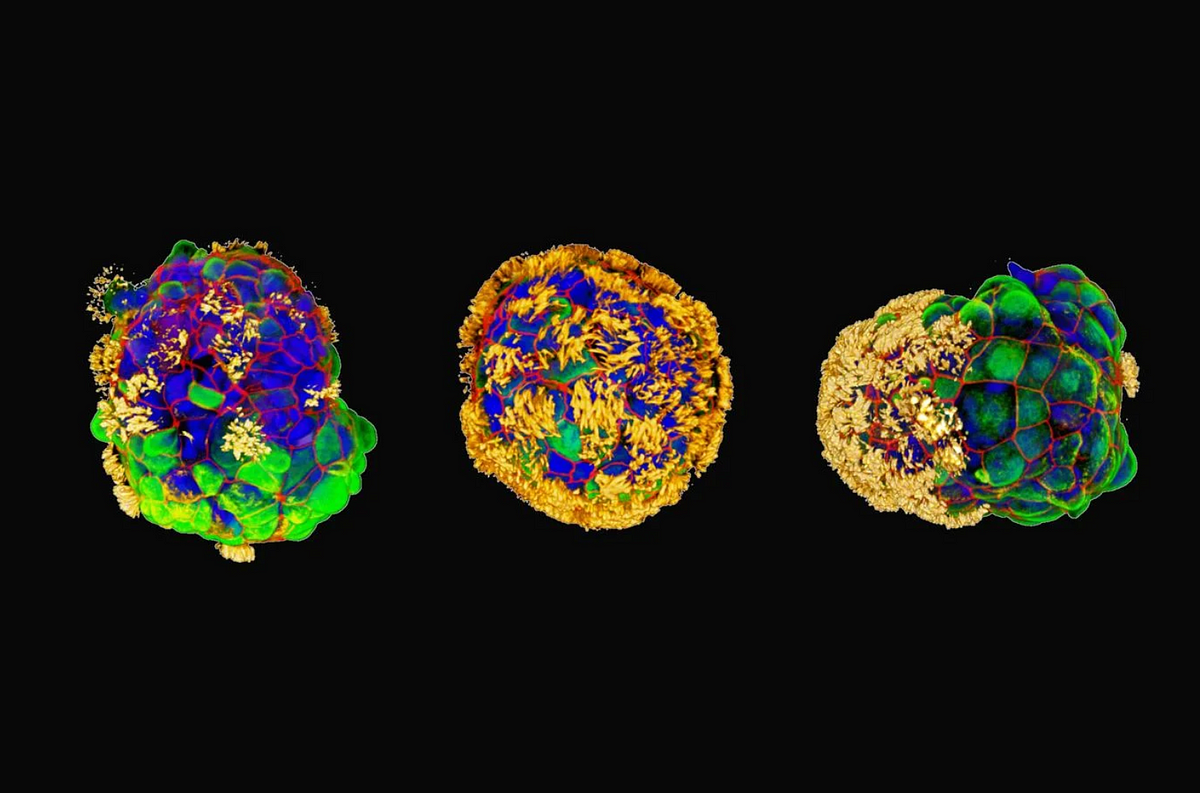
Now, Levin and his colleagues at Tufts University have extended their research by developing similar “robot-like” entities using human cells, which they’ve named anthrobots.
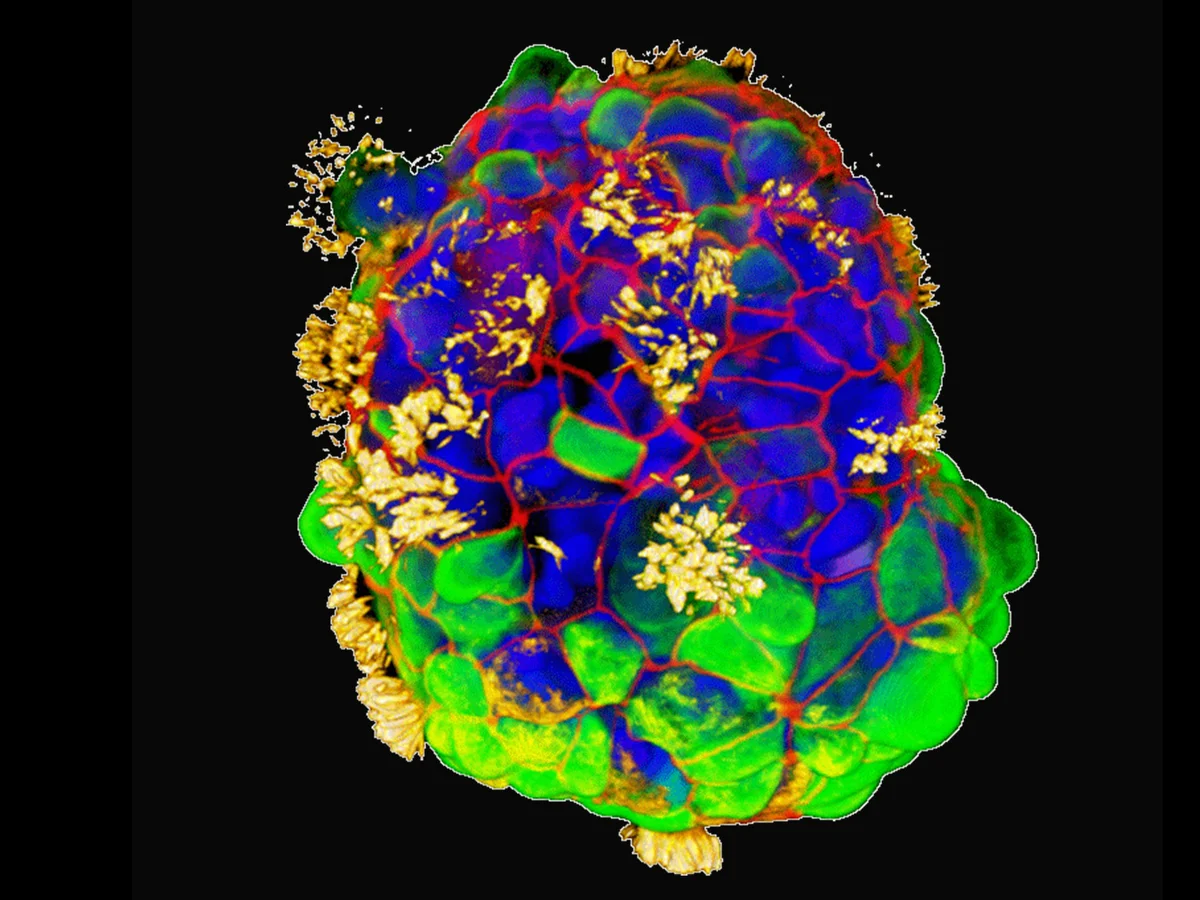
Published in Advanced Science, their study reveals that anthrobots move using hair-like protein appendages called cilia, coordinating their motion to propel through fluids effectively.
Beyond swimming, anthrobots display distinct shapes and behaviors akin to strains or groups within a species.
Intriguingly, the Tufts team observed that anthrobots could initiate basic wound healing in layers of human cells, hinting at potential medical applications.
While some scientists remain skeptical about classifying these human cell constructs as true “robots,” questioning their novelty compared to the original xenobots, Levin advocates for a paradigm shift.
He suggests viewing these cell clusters not merely as tools for studying human biology, but as independent entities with unique forms and behaviors.
This perspective opens doors to using them as a versatile “biorobotics platform” for diverse applications, including tailored medical interventions aimed at tissue repair and beyond.