AstraZeneca (AZN) has submitted a request to the FDA to approve its nasal flu vaccine, FluMist, for home use, aiming to make it the first self-administered vaccine ever authorized.
The pharmaceutical company announced on Tuesday morning that its supplemental license application has been accepted by the FDA, seeking approval for home use in individuals aged 2-49.
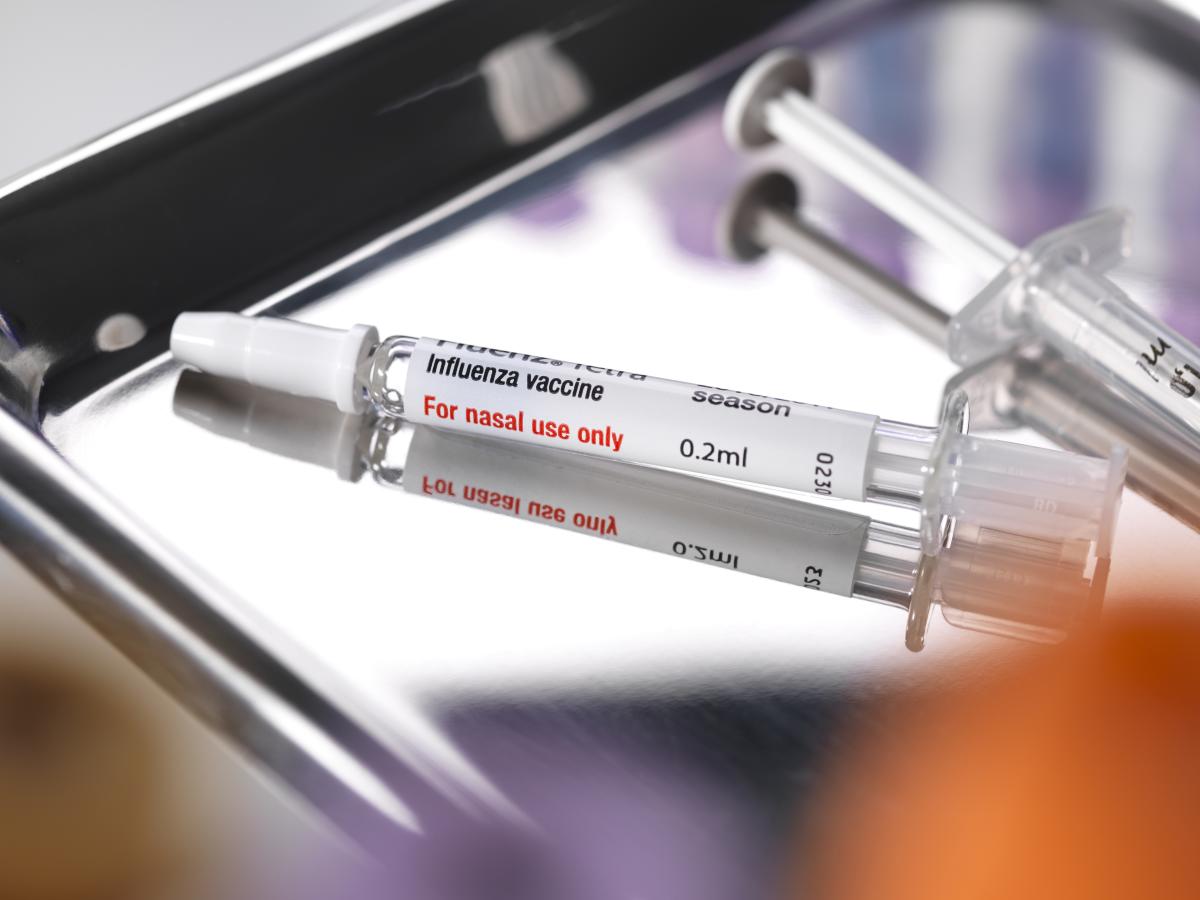
While FluMist has been available for two decades, it has previously only been administered in pharmacy or clinical settings.
AstraZeneca anticipates a decision from the FDA by the first quarter of 2024.
According to the company, adults aged 18 and older would be able to self-administer the single-use nasal syringe and could also administer the vaccine to children aged 2-17, pending approval.
Lisa Glasser, head of US medical affairs, vaccines, and immune therapies at AstraZeneca, explained to Yahoo Finance that this move reflects lessons learned from the COVID-19 pandemic.
“We provided nasal swabs for COVID testing, which people found easy to use and continue to utilize,” Glasser said.
The company expressed confidence that individuals will find the nasal syringe easy to use, noting it will be distinct from nasal spray medications, coming in a single syringe with clear instructions.
Ensuring the vaccine’s temperature stability during shipping to patients remains a critical aspect.
AstraZeneca is preparing logistics to maintain the required temperature controls, although specifics about packaging and shipment are yet to be finalized.
“People will be able to order FluMist through a website or an online pharmacy partner, it’ll be shipped to their home in packaging that maintains optimal temperature controls for the vaccine,” Glasser detailed.
AstraZeneca pursued this initiative due to low flu vaccine uptake during annual seasons, even with efficacy rates around 50% this year, Glasser acknowledged.
“While flu vaccines aren’t perfect, they help mitigate the impact of the disease. Getting vaccinated is better than not getting vaccinated,” she emphasized.
Furthermore, this development paves the way for other diseases such as COVID-19. Glasser mentioned ongoing efforts to develop a live attenuated SARS-CoV-2 vaccine for intranasal delivery.
Given that respiratory diseases typically enter through the nose, delivering vaccines at this point of entry is considered ideal, Glasser highlighted.
