The Centers for Disease Control and Prevention (CDC) recommended on Thursday that adults aged 60 and older should consider receiving a single dose of RSV vaccines from Pfizer and GSK after consulting with their healthcare providers.
CDC Director Rochelle Walensky approved the recommendation, which was initially proposed by an advisory panel of external experts last week.
The endorsement advises seniors to discuss with their doctors whether getting vaccinated is appropriate for them.
The CDC indicated that these vaccines are anticipated to be available to the public in the upcoming fall, coinciding with the seasonal increase in respiratory syncytial virus (RSV), COVID-19, and influenza transmission.
“These vaccines offer an opportunity to help safeguard older adults from severe RSV illness during a period when multiple respiratory infections are expected to be circulating,” the CDC stated in a release.
RSV is a prevalent respiratory infection that typically causes mild cold-like symptoms but can lead to more severe cases in older adults and young children.
According to CDC data, RSV annually causes between 6,000 to 10,000 deaths among seniors and several hundred deaths in children under five years old.
Walensky’s decision follows the U.S. Food and Drug Administration’s approval of these vaccines a month ago, marking them as the world’s first authorized vaccines against RSV.
Representatives from Pfizer and GSK did not immediately respond to requests for comment.
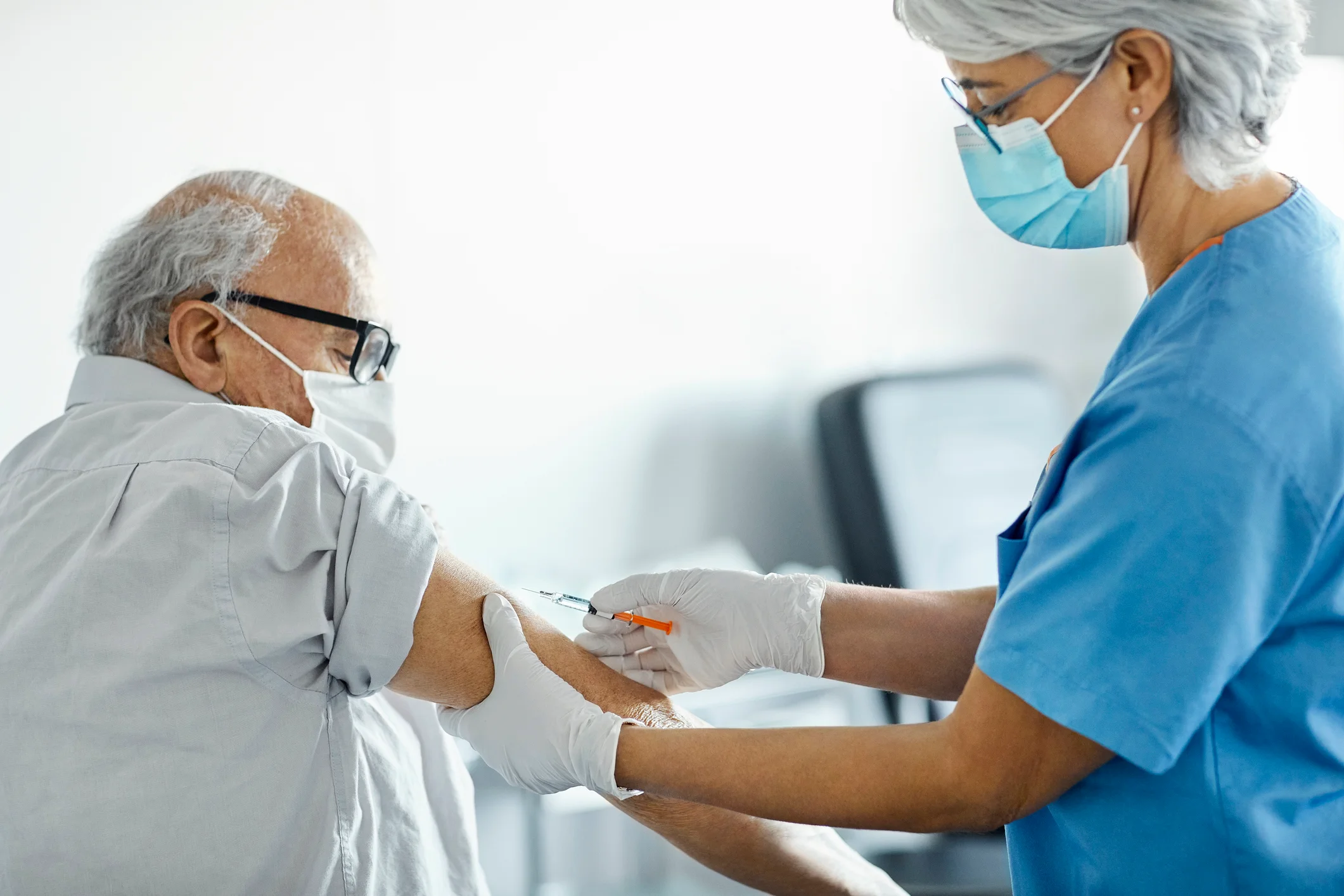
Last week, both companies revealed late-stage clinical data demonstrating that their respective vaccines generally maintain protection against RSV following one season of virus circulation, typically occurring in the U.S. from October to March.
However, the advisory panel expressed concerns regarding the lack of efficacy data in subgroups of elderly individuals who are at the highest risk of severe RSV.
Dr. Michael Melgar, a CDC medical officer who reviewed data from both vaccines, noted during the advisory panel meeting that adults aged 75 and older and those with underlying medical conditions were underrepresented in phase 3 clinical trials conducted by both companies.
Seniors with weakened immune systems were entirely excluded from these trials.
Both Pfizer and GSK stated that studies involving these vulnerable populations are ongoing.
The CDC panel also raised concerns about the pricing of the vaccines, which could potentially limit accessibility for some Americans.
GSK announced pricing between $200 and $295 for its vaccine, while Pfizer set its price between $180 and $270. However, both companies did not commit to these prices.
Additionally, Pfizer has developed a vaccine designed to protect newborns from RSV.
An FDA advisory panel supported this vaccine last month but expressed safety concerns regarding potential associations with premature births. The FDA is expected to make a final decision on this vaccine in August.