An additional fatality has occurred in an outbreak linked to contaminated eye drops, with an increase in reports of individuals experiencing vision loss.
The death toll has now reached four, as reported by the Centers for Disease Control and Prevention (CDC) on Friday, initially covered by ABC News. While one death was confirmed in Washington state, no details were provided about the other victims.
Furthermore, the number of individuals who have gone blind has risen to at least 14, up from eight reported in the previous update in March. Four people have undergone surgical removal of their eyeballs, a figure that has remained unchanged.
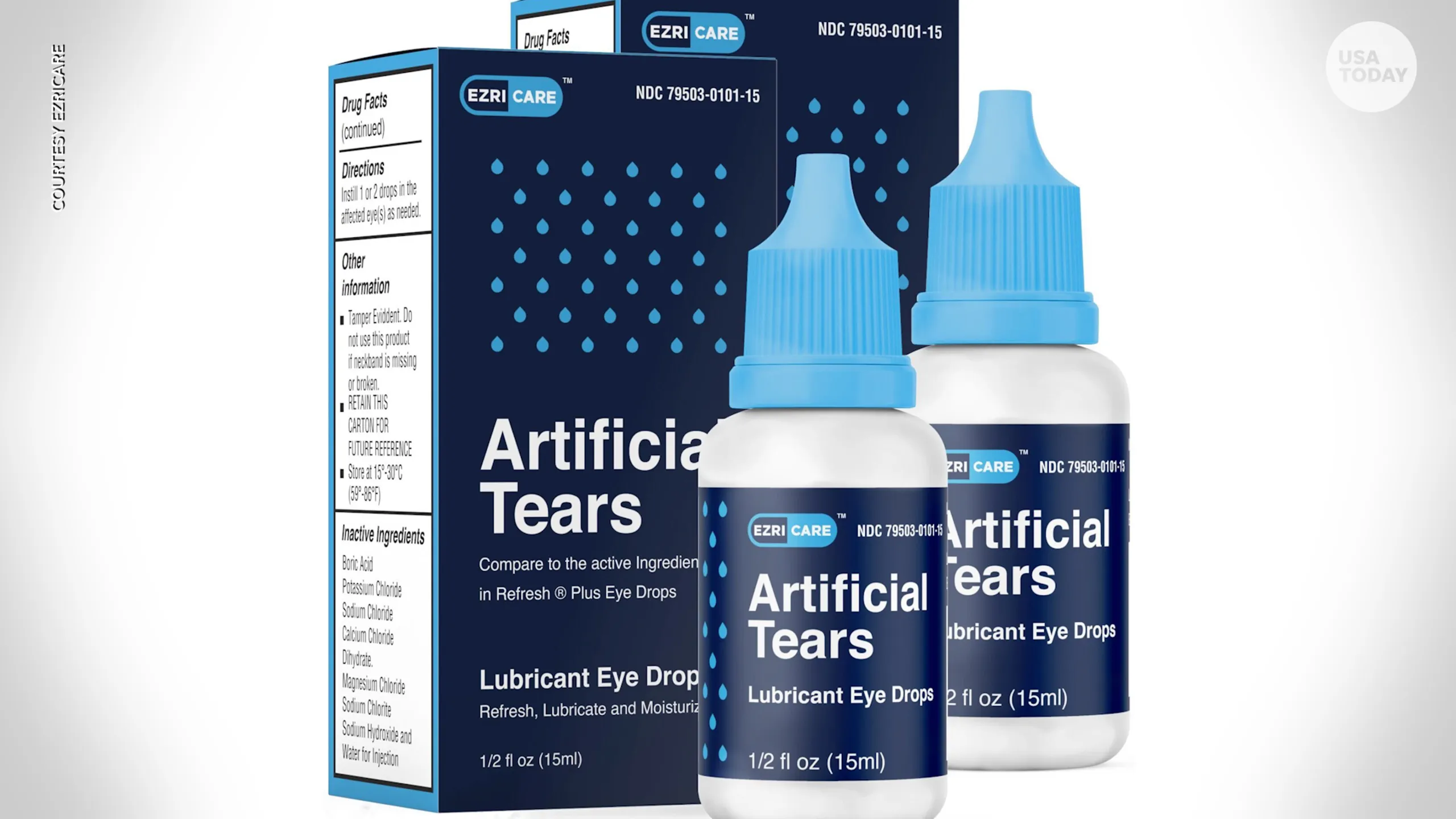
Patients reported using various brands of artificial tears, but most cases have been associated with EzriCare and Delsam Pharma eye drops, manufactured by Global Pharma Healthcare based in India. These products were contaminated with an antibiotic-resistant strain of Pseudomonas aeruginosa, a virulent bacterium.
Pseudomonas bacteria are commonly found in the environment, with P. aeruginosa known for causing infections in humans.
The bacterium is prevalent in healthcare settings due to poor hygiene practices, such as inadequate handwashing and improper cleaning of medical equipment and surfaces.
P. aeruginosa is resistant to multiple antibiotics and has caused approximately 32,600 infections among hospitalized patients in the U.S., resulting in an estimated 2,700 deaths, according to CDC data.

Notably, the strain implicated in this outbreak had never been previously reported in the United States, as highlighted in the CDC’s latest update.
As of May 15, a total of 81 people across 18 states have been infected with P. aeruginosa, marking an increase of 13 cases since the last update.
Symptoms of infection include discharge from the eye (yellow, green, or clear), eye pain or discomfort, red eyes or eyelids, sensation of something in the eye, heightened sensitivity to light, and blurry vision.
In February, the U.S. Food and Drug Administration (FDA), in collaboration with the CDC, issued a warning advising against the purchase of EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears due to potential bacterial contamination.
Subsequently, Global Pharma Healthcare voluntarily recalled both products and notified distributors to cease distribution. Additionally, Global Health Pharma recalled Delsam Pharma’s Artificial Ointment.
The CDC and FDA urge anyone still in possession of these brands to discontinue use immediately and discard the products. None of the recalled items are available for purchase online.
Of the 13 new cases reported to the CDC, specimens were collected from six patients before the February recall.
According to the CDC’s update, these cases were confirmed post-recall due to the time required for testing to identify the outbreak strain and retrospective reporting of infections.
Among the seven patients with specimens collected after the recall, they either resided in long-term care facilities with other known cases or had used a recalled brand of artificial tears.