A Texas judge is poised to issue a critical ruling in a high-profile case that challenges the Food and Drug Administration’s approval of the abortion pill mifepristone.
The lawsuit, filed by a group of doctors who oppose abortion known as the Alliance for Hippocratic Medicine, is an unprecedented case. Judge Matthew Kacsmaryk of the U.S. Northern District of Texas has several potential courses of action.
He could mandate the FDA to completely remove mifepristone from the U.S. market. Alternatively, Kacsmaryk might order the agency to implement stricter access restrictions without halting sales entirely. He could also rule in favor of the FDA, though anti-abortion groups would likely appeal.
During oral arguments in Amarillo on Wednesday, Kacsmaryk informed the attorneys involved that he would issue his decision “as soon as possible.”
Abortion rights groups and legal experts anticipate that the judge will rule against the FDA in some capacity. Kacsmaryk joined the court in 2019 after being appointed by former President Donald Trump.
His nomination faced unanimous opposition from Senate Democrats and Republican Susan Collins of Maine due to concerns about his views on abortion and LGBTQ rights.
Wendy Davis, senior advisor at Planned Parenthood Texas Votes, stated at a news conference on Wednesday that abortion rights activists are bracing for the worst.
A court order blocking mifepristone sales would most significantly impact states where abortion remains legal, according to Carrie Flaxman, who leads litigation at the Planned Parenthood Federation of America.
Rachel Rebouche, a reproductive health law expert at Temple University, mentioned that an order blocking sales would lead to confusion, as further litigation over the legality of such an order would ensue.
If Kacsmaryk issues an order to withdraw mifepristone from the market, the specifics of the ruling will be crucial. The impact of his decision will depend on the wording of the order and how the FDA responds.
“There are a lot of ways the court could effectuate a decision in our favor,” said Erik Baptist, who represents the Alliance for Hippocratic Medicine and serves as senior counsel at the Alliance Defending Freedom, another anti-abortion group.
During a news conference on Thursday, Baptist indicated that the judge could overturn the FDA’s approval immediately or order the agency to begin the process of removing mifepristone from the U.S. market.
“But how the court effectuates in terms of timing – does it go into effect immediately, does it go into effect in 30 days, again that’s within the court’s discretion,” Baptist said.
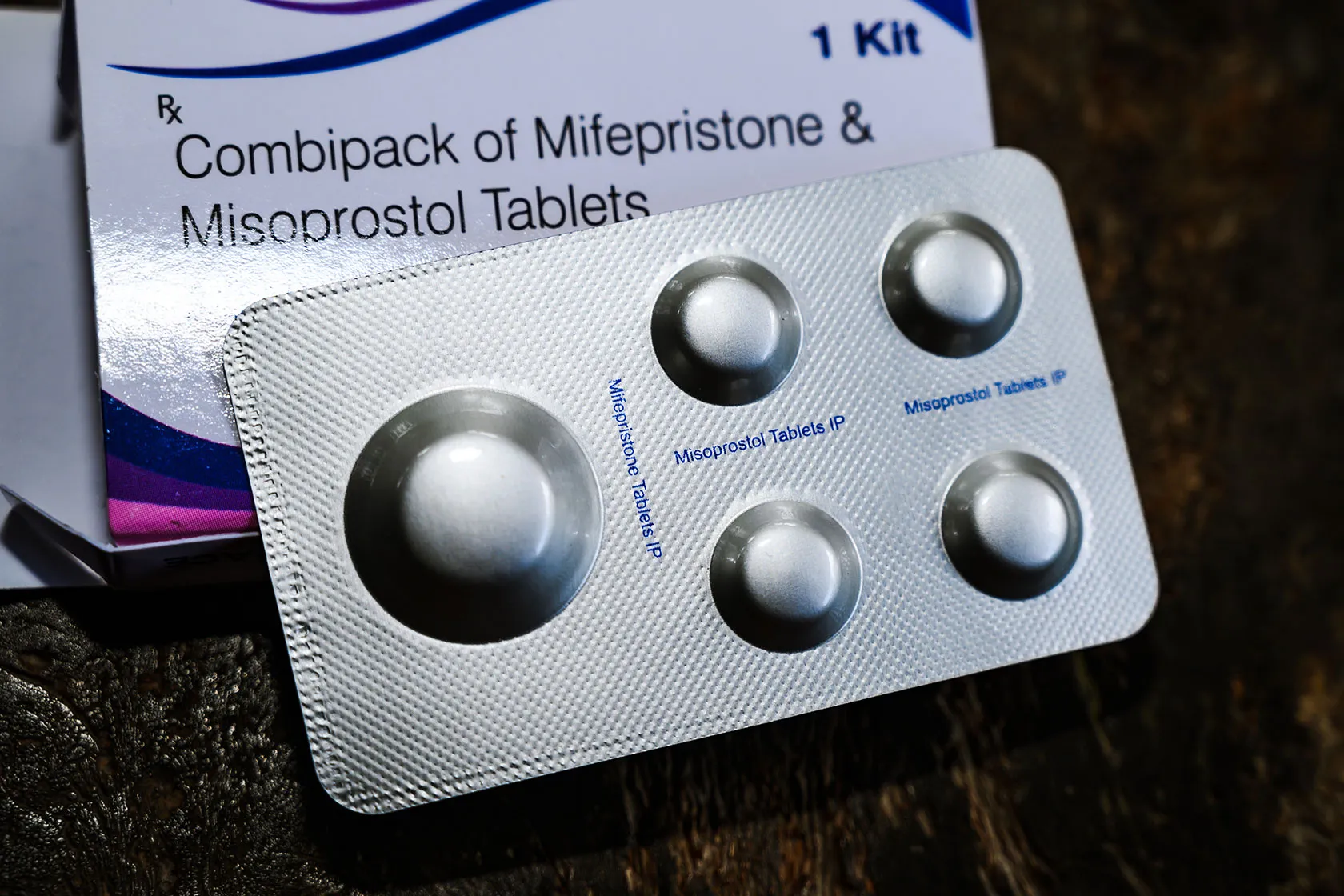
Rebouche noted that the judge might issue a ruling requiring the FDA to start the process of withdrawing mifepristone while also suspending the drug from the market during this period.
Should Kacsmaryk order an immediate withdrawal of mifepristone, the Biden administration would likely request a pause on the decision pending its appeal, according to Glenn Cohen, a health law expert at Harvard.
If Kacsmaryk refuses, the administration will take the case to the 5th U.S. Circuit Court of Appeals.
“My guess is stay papers are already drafted. Someone will put them before the court within hours of the decision,” said Cohen, who formerly served as a lawyer in the Justice Department’s civil division.
Cohen, Rebouche, and 17 other drug law experts, in a court filing supporting the FDA, argued that ordering an immediate withdrawal of mifepristone would conflict with federal law.
They stated that the authority to withdraw a drug lies with the FDA commissioner, who bases such decisions on scientific evidence of the drug’s safety and efficacy. The manufacturer, Danco Laboratories in this case, is also entitled to a hearing during the process.
“The FDA would argue the court cannot withdraw the drug — the FDA has to withdraw the drug and the court is preempted by federal statute from withdrawing the drug,” said Rebouche.
If Kacsmaryk opts not to order an immediate withdrawal but instead directs the FDA to initiate its formal process to remove the drug, the agency can use the process to delay, provided the judge does not suspend the approval during that time.
“The withdrawal of a drug from the market when the FDA follows those procedures takes months if not years, so the FDA could try to draw out the process a little bit longer to keep the drug on the market in the meantime,” said Allison Whelan, an FDA law expert at Georgia State University.
“The FDA does not like its scientific expertise and legitimacy to be called into question,” said Whelan, who also signed the filing supporting the FDA.
The agency also has enforcement discretion, allowing it to choose not to pursue companies selling unapproved drugs, Whelan noted.
Mifepristone is also approved for treating Cushing’s syndrome. Some clinics might decide to prescribe the pill off-label for abortions, she said.
Mifepristone is used in a two-drug regimen with another medication, misoprostol. Baptist from the Alliance Defending Freedom stated during the Thursday news conference that the lawsuit targets only the approval of mifepristone.
The World Health Organization recommends misoprostol as a standalone method to terminate a pregnancy. Although the FDA has not approved misoprostol alone for abortions, clinics plan to use it as an alternative to mifepristone.
The American College of Obstetricians and Gynecologists recommends misoprostol as an alternative for early abortions if mifepristone is unavailable, though it is not as effective as the two-drug regimen.
Kacsmaryk could also opt to impose stricter restrictions on mifepristone distribution rather than halting sales entirely.
In January, the FDA permanently lifted the requirement that patients obtain mifepristone in person from a certified healthcare provider, allowing telehealth appointments and mail delivery.
The Alliance for Hippocratic Medicine has asked the judge to reinstate FDA restrictions that have been eased over the years, citing a federal statute from 1873 called the Comstock Act, which bans sending abortion medication via mail.
Rebouche pointed out that the Comstock Act has not been enforced in decades, but there is a possibility that the judge could attempt to revive the statute to compel the FDA to reimpose the requirement that patients obtain mifepristone in person.
